
Prof. Hartung is currently Professor of Neurology at Heinrich-Heine-University Düsseldorf, Honorary Professor at Brain and Mind Center, University of Sydney, Visiting Professor at Medical University Vienna and Palacky University Olomouc. He was chairman of the Department of Neurology, Heinrich-Heine-University Düsseldorf from 2001-2020, director of the Center for Neurology and Neuropsychiatry from 2012-2020 and director of the Department of Conservative Medicine from 2012-2019.
Prof. Hartung’s clinical and translational research interests are in the field of basic and clinical neuroimmunology and in particular multiple sclerosis and immune neuropathies, development of new immunological, neuroprotective and neural repair promoting strategies. He has (co-)authored more than 950 articles in peer-reviewed journals and 100 book chapters. He has been involved as member of the Steering Committee in numerous international multicentre therapeutic phase 2 and 3 trials in multiple sclerosis, Guillain-Barré Syndrome and CIDP.
He was President of ECTRIMS and has served/ serves amongst others on the executive boards of the European Charcot Foundation, the European Neurological Society, and the International Multiple Sclerosis Cognition Society (IMSCOGS). He is/was also member of the Editorial Board of a number of international journals. Prof. Hartung is a Fellow of the AAN and EAN, and has been chair/ member of the management group of the EAN scientific panels on general neurology and multiple sclerosis. He is Corresponding and Honorary Fellow of several international societies.
Conflict of Interest Statement:
Hans-Peter Hartung has received fees for consulting, speaking, and serving on steering committees from Bayer Healthcare, Biogen, GeNeuro, MedImmune, Merck, Novartis, Opexa, Receptos Celgene, Roche, Sanofi Genzyme, CSL Behring, Octapharma, Teva, TG Therapeutics, and Viela Bio, with approval by the Rector of Heinrich-Heine University.
Posted on
Previous Article
« ACR presents new guideline for lupus nephritis Next Article
Introduction to the ECTRIMS 2024 Conference Report »
« ACR presents new guideline for lupus nephritis Next Article
Introduction to the ECTRIMS 2024 Conference Report »
Table of Contents: ECTRIMS 2024
Featured articles
Diagnosis, Biomarkers, and Phenotypes
Revised McDonald criteria allow earlier and more precise MS diagnosis
Approaches to RIS and MS converge
AI versus clinicians: who diagnoses MS faster and better?
Blood markers predict MS progression
Gut microbiota modulate inflammation and cortical damage
Risk factors and importance of persistent PIRA
Vitamin D supplementation in progressive MS should be medically supervised
Treatment: Strategies
Encouraging real-world results of AHSCT to treat aggressive MS
CAR T-cell therapy in MS: in its infancy but highly anticipated
B cell-tailored dosing of ocrelizumab shows good results
First-line moderate-efficacy DMTs show similar efficacy
Treatment: Trials
Tolebrutinib slows disability worsening in relapsing MS
Frexalimab shows favourable safety and efficacy in OLE
Good safety of ozanimod over up to 8 years of treatment
Tolebrutinib slows disability in non-relapsing SPMS
High-dose simvastatin does not slow disability progression in SPMS
Comorbidity Risks and Pregnancy
High genetic burden for depression associated with MS disease activity
More comorbidity is associated with worse clinical outcomes in MS
Transfer of ocrelizumab into breastmilk is negligible
NMOSD/MOGAD
Ineffective response to EBV in MS not seen in similar diseases
Comparative effectiveness and safety of DMTs in NMOSD
Age, time, and treatment determine relapse risk in MOGAD
Related Articles
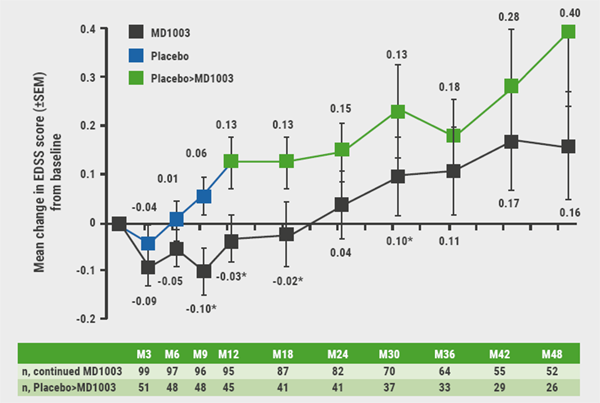
July 30, 2019
Biotin, ocrelizumab, and ibudilast in progressive MS
September 10, 2020
Sustained benefits of avalglucosidase alfa in late-onset Pompe disease
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

