Amantadine may display anti-viral activity against the SARS-CoV-2 virus, but even more important are its potential CNS effects including neurotransmitter modulation and anti-inflammatory activity, stated Prof. Konrad Rejdak (Medical University of Lublin, Poland) [1].
The phase 3 COV-PREVENT trial (NCT04854759) assessed the efficacy and safety of amantadine in the prevention of COVID-19 progression to acute respiratory failure and neurological complications. The study enrolled 200 patients from 8 Polish centres who were positive for SARS-CoV-2 and had 1 or more risk factors for clinical complications of the infection, such as obesity, hypertension, or pulmonary disease. Exclusion criteria included an infection severe enough to meet the study's primary endpoint of clinical worsening, and a WHO score of 4 or more, implying oxygen therapy during hospitalisation.
Of 200 enrolled participants, 111 were randomised to amantadine (n=54) or placebo (n=57), administered orally at a dose of 100 mg twice daily (morning and noon) for 14 days. The mean age was 48 years, 53% were men, and 54% of participants were hospitalised. The randomised phase was followed by an open-label phase (days 15–210), in which participants were offered an additional 14 days of treatment with amantadine at 100 mg once daily. The primary outcome measure was clinical worsening: moderate/severe dyspnoea, drop in O2-saturation, and/or reaching a WHO score of 4 or more at day 15.
According to Prof. Rejdak, SARS-CoV-2 generally remained mild between days 1–15. In the amantadine and placebo groups, 0 and 4 (8%) patients progressed to WHO score >4, respectively; 3 (6.1%) and 4 (8%) progressed to WHO=4. “However, more patients progressed to a higher WHO score in the placebo group than in the amantadine group”, observed Prof. Rejdak, adding that these differences were not significant. There was, however, a significant difference in the number of recovered patients (WHO=0) in favour of amantadine versus placebo (63.3% vs 40%; see Table).
Table: Disease progression/death (WHO≥4) or recovery (WHO=0) from COVID-19 in observation between days 1–15, and survival in extended follow-up until day 210 [1]
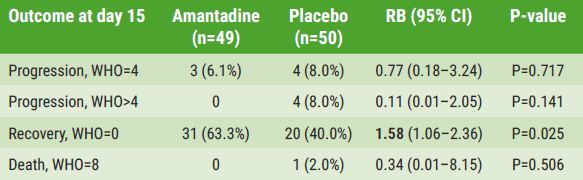
“In a logistic regression analysis, treatment with amantadine was significantly associated with favourable outcomes (P=0.028),” Prof. Rejdak noted. In patients with 1–2 risk factors, the relative benefit was 1.29 (95% CI 0.83–1.99); in patients with 3–4 risk factors, the relative benefit was higher at 4.09 (95% CI 1.15–14.57). Neurological outcomes improved as well in the amantadine group, including depression, sleepiness, sense of taste and smell, and fatigue. Amantadine was well tolerated and was not associated with more side effects than placebo.
- Rejdak K, et al. Amantadine in early COVID-19: a randomised, placebo-controlled, double-blind, phase 3 trial. EAN 2023 Annual Meeting, 1–4 July, Budapest, Hungary.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« SARS-CoV-2 vaccination in CIDP and MMN: more benefit than harm Next Article
Intensive BP reduction associated with smaller haematoma »
« SARS-CoV-2 vaccination in CIDP and MMN: more benefit than harm Next Article
Intensive BP reduction associated with smaller haematoma »
Table of Contents: EAN 2023
Featured articles
Letter from the Editor
Alzheimer’s disease and dementia: the road towards proactive and preventive care
Overarching Theme: Big Data
Contribution of genomics and genetics to personalised medicine
How big data can boost care for neurodegenerative disorders
COVID-19
Amantadine in early COVID-19 enhances recovery
SARS-CoV-2 vaccination in CIDP and MMN: more benefit than harm
Cerebrovascular Disease and Stroke
Intensive BP reduction associated with smaller haematoma
Cognition and Dementia
Towards cell biology of Alzheimer’s disease
Epilepsy
Minimising co-medication optimises cenobamate efficacy in drug-resistant epilepsy
Headache and Pain
GLP-1 agonists induce weight loss and alleviate headache in idiopathic intracranial hypertension
Cannabis-based medicine does not beat placebo in central neuropathic pain
80% of patients reverse from chronic to episodic migraine on anti-CGRP antibodies
Multiple Sclerosis
Which patients can initially be treated with platform DMT?
Retinal layer thickness predicts disability accumulation in early RMS
Withdrawing DMF in early pregnancy does not increase relapse risk in pregnant patients with MS
Immunosenescence and MS: relevance to immunopathogenesis and treatment
Sleep Disorders
Nightmares during childhood linked to cognitive decline later in life
Sleep changes contribute to the pathogenesis of neurodegenerative diseases
Miscellaneous
EAN guidelines on the management of ALS
What neurologists should know about bladder and sexual problems
Laughing gas abuse often leads to polyneuropathy, myelopathy, and encephalopathy
Related Articles
September 7, 2023
What neurologists should know about bladder and sexual problems
September 7, 2023
EAN guidelines on the management of ALS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

