sNfL is a known biomarker to evaluate therapy effectiveness and disease activity in MS patients. The phase three REFLEX trial (NCT00404352) showed that treatment with subcutaneous interferon-beta-1a (scIFNβ-1a) delayed the onset of McDonald 2005 MS in patients with a first clinical demyelinating event. This treatment also reduced sNfL levels from 6 months post-baseline. The current post-hoc analysis of this trial analysed whether baseline sNfL levels in patients treated with scIFNβ-1a could predict the onset of McDonald 2005 MS. Results were presented by Prof. Jens Kuhle (University of Basel, Switzerland).
A two-year follow up of patients in the scIFNβ-1a thrice-weekly (n=171), once-weekly (n=175), and placebo (n=171) groups showed that baseline levels of sNfL predicted the conversion to McDonald 2005 MS in all groups. Patients with high sNfL levels at baseline (>26.1 μg/mL) have an increased risk of developing definite MS within a 2-year timeframe. sNfL subgroup analysis demonstrated that treatment with scIFNβ-1a delayed MS onset in both patients with low and high baseline levels of sNfL. Other findings from this study demonstrated that a younger age, multifocal first clinical demyelinating event, and the number of T1 and T2 lesions predicted the onset of definite MS in all groups of the REFLEX trial.
- Kuhle J, et al. Baseline Serum Neurofilament Light Chain Levels Predict Conversion to McDonald 2005 Multiple Sclerosis (MS) Within 2 Years of a First Clinical Demyelinating Event in Patients with MS. P5.080, AAN 2021 Virtual Congress, 17-22 April.
Posted on
Previous Article
« No link between antidepressants and intracerebral haemorrhage in stroke patients Next Article
No effect of foetal antiseizure exposure on neurodevelopmental outcomes at age 3 »
« No link between antidepressants and intracerebral haemorrhage in stroke patients Next Article
No effect of foetal antiseizure exposure on neurodevelopmental outcomes at age 3 »
Related Articles

June 16, 2021
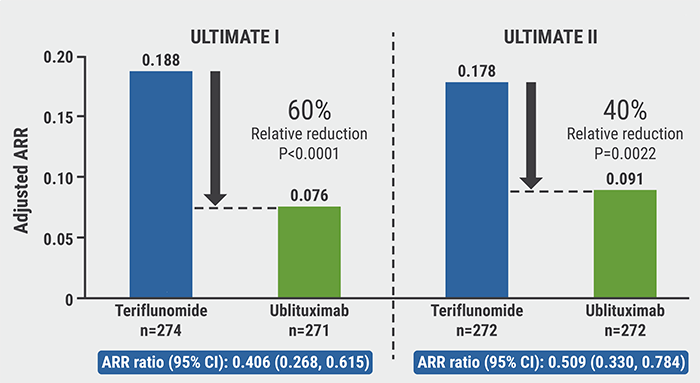
Ublituximab versus teriflunomide in relapsing MS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

