https://doi.org/10.55788/c407f4e9
For more than 2 decades, chemoradiotherapy followed by brachytherapy has been the standard of care for patients with locally advanced cervical cancer (FIGO IB3–IVA). Although the local control of disease has increased over time, up to 30% of patients eventually relapse and die from metastatic disease. A recent phase 2 study showed the feasibility and a good response rate of induction chemotherapy using weekly paclitaxel and carboplatin for 6 cycles, immediately followed by standard chemoradiotherapy [1].
The current phase 3 INTERLACE trial (EudraCT: 2011-001300-35) randomised 500 participants with locally advanced cervical cancer (stage IB1 node positive–IVA) to standard chemoradiotherapy or induction chemotherapy followed by standard chemoradiotherapy. The primary endpoints were PFS and OS. Results were presented by Dr Mary McCormack (University College London, UK) [2].
About 75% of enrolled participants presented with stage IIA or IIB disease, 82% showed squamous histology and almost 60% of participants and tumours were node-negative. The adherence to induction chemotherapy was high, with more than 90% of participants having received at least 5 cycles of induction chemotherapy. Also, more than 90% of participants adhered to radiotherapy in both study arms. Induction chemotherapy substantially increased both PFS and OS. At the 5-year follow-up, 73% of participants in the induction arm were progression-free versus 64% in the control arm (HR 0.65; 95% CI 0.46–0.91; P=0.013). At the same follow-up time point, 86% of participants in the induction arm were still alive versus 80% in the control arm (HR 0.61; 95% CI 0.40–0.91; P=0.04, see Figure). “OS in the control arm was similar to that in the recent literature,” remarked Dr McCormack. Total local relapse rates after 5 years were 16% in both arms. In contrast, the total distant relapse rate after 5 years was 12% in the induction arm versus 20% in the control arm.
Figure: Overall survival results from the INTERLACE trial over time [2]
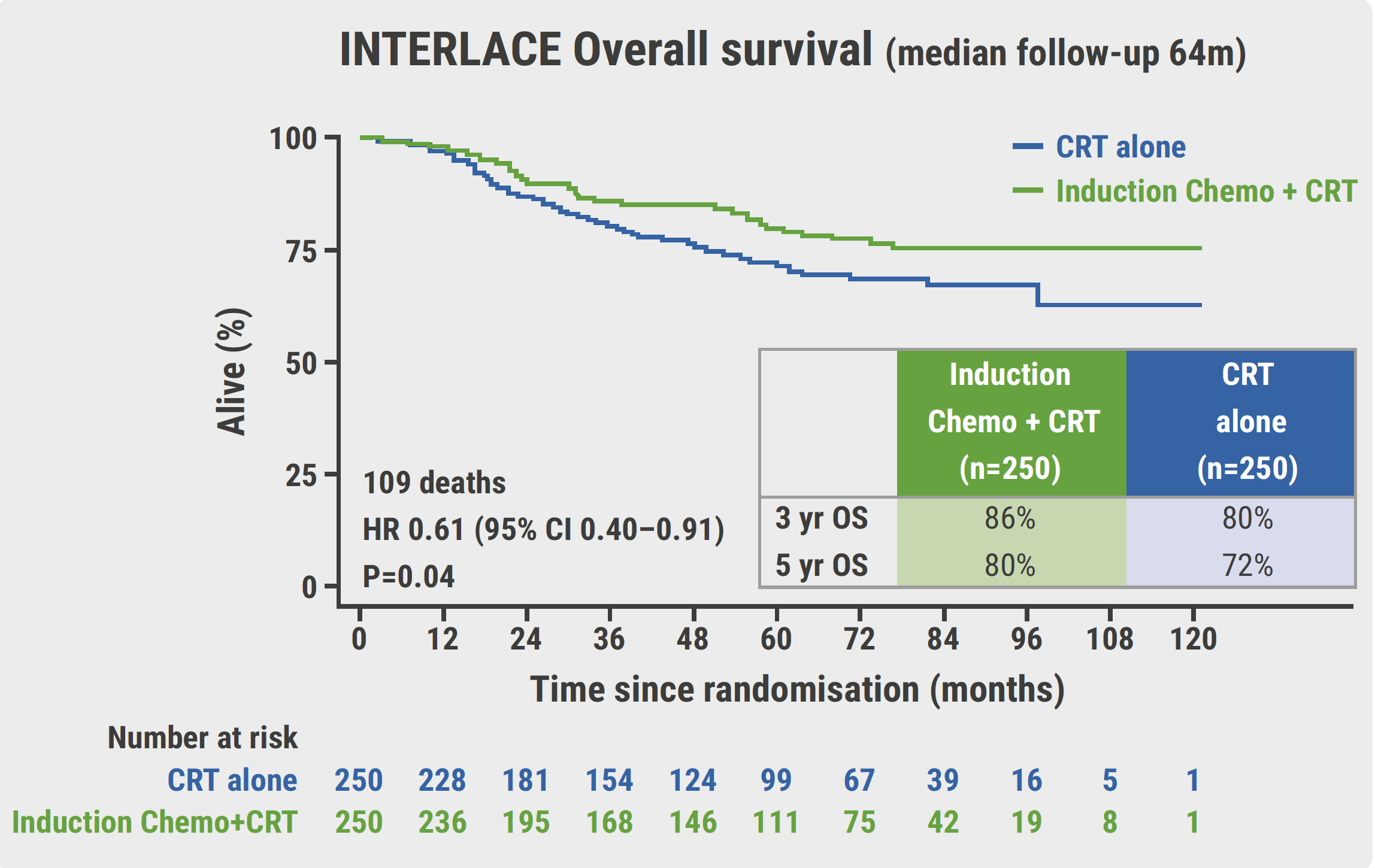
CI, confidence interval; CRT, chemoradiotherapy; FU, follow-up; HR, hazard ratio; OS, overall survival; Yr, year.
“In conclusion, the INTERLACE trial showed that short induction chemotherapy with paclitaxel and carboplatin can significantly improve PFS and OS and decrease distant relapses. This induction protocol is feasible across different healthcare settings and should be considered the new standard in locally advanced cervical cancer,” summarised Dr McCormack.
- McCormack M, et al. Br J Cancer. 2013;108:2464–2469.
- McCormack M, et al. A randomised phase III trial of induction chemotherapy followed by chemoradiation compared with chemoradiation alone in locally advanced cervical cancer: The GCIG INTERLACE trial. Abstract LBA8, ESMO 2023, 20–24 October, Madrid, Spain.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Neoadjuvant immune checkpoint blockade safe and effective in MMRd endometrial cancer Next Article
Addition of atezolizumab to chemotherapy and maintenance PARP inhibitor has no benefit in ovarian cancer »
« Neoadjuvant immune checkpoint blockade safe and effective in MMRd endometrial cancer Next Article
Addition of atezolizumab to chemotherapy and maintenance PARP inhibitor has no benefit in ovarian cancer »
Table of Contents: ESMO 2023
Featured articles
The importance of detecting early NSCLC
Breast Cancer
Benefit of pembrolizumab in TNBC remains after 5 years of follow-up
Addition of pembrolizumab promising in early-stage high-risk ER+/HER2- breast cancer
Long-term air pollution exposure at both residential and workplace locations increases breast cancer risk
Third-line datopotamab deruxtecan improves progression-free survival in previously treated metastatic HR+/HER2- breast cancer compared with chemotherapy
Colorectal Cancer
Neoadjuvant nivolumab/relatlimab demonstrates 100% pathological response in MMRd colon cancer
Selective KRASG12C inhibitor sotorasib leads to superior PFS in colorectal cancer
Postoperative ctDNA predicts survival in colorectal cancer
Overall survival in patients with initially unresectable colorectal liver metastases does not depend on choice of induction regimen
Lung Cancer
Perioperative nivolumab boosts event-free survival in NSCLC
Selective RET inhibitor selpercatinib doubles progression-free survival in RET-mutated NSCLC
Dato-DXd outperforms docetaxel in previously treated patients with metastatic NSCLC
First-line and second-line benefit of amivantamab in advanced, EGFR-mutated NSCLC
Upper Gastro-Intestinal Cancer
Perioperative durvalumab/FLOT improves pCR in gastric cancer
Active surveillance after neoadjuvant chemoradiotherapy in oesophageal cancer
FOLFIRINOX equals gemcitabine-based chemoradiotherapy in neoadjuvant setting for pancreatic cancer
Modified FLOT regime outperforms FOLFOX in advanced/metastatic gastric/gastroesophageal junction adenocarcinoma
Melanoma
Lifileucel induces a durable response in heavily pretreated mucosal melanoma
Darovasertib/crizotinib combination: a potential first-line therapy in metastatic uveal melanoma
Genito-Urinary Cancers
Two potential new first-line standards of care in metastatic urothelial cancer
LuPSMA and enzalutamide: a promising combination
No benefit of erdafitinib over pembrolizumab in urothelial cancer second-line therapy
Gynaecological Cancers
Addition of atezolizumab to chemotherapy and maintenance PARP inhibitor has no benefit in ovarian cancer
Short-induction chemotherapy improves survival in advanced cervical cancer
Neoadjuvant immune checkpoint blockade safe and effective in MMRd endometrial cancer
Featured Interviews
Can radiotracers predict response to PD-L1 inhibitors in early NSCLC?
The importance of detecting early NSCLC
Related Articles

September 16, 2021
U.S. FDA approves Takeda’s lung cancer therapy
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

