New exploratory subgroup findings from the TAGS study (NCT02500043) were presented by Prof. Josep Tabernero (Vall d'Hebron Institute of Oncology, Spain), specifically focused on results for third- and later-line treatment with trifluridine/tipiracil [1]. The TAGS study investigated the efficacy and safety of TAS-102 (trifluridine, an antineoplastic thymidine-based nucleoside analogue, and tipiracil, a thymidine phosphorylase inhibitor) plus best supportive care (BSC) compared with placebo plus BSC in patients with metastatic gastric cancer (including adenocarcinoma of the gastroesophageal junction) that was refractory to standard treatments.
Patients were randomised in a 2:1 ratio to receive TAS-102 at a 35 mg/m2 twice daily on days 1 to 5 and 8 to 12 of each 28-day cycle (n=337) or placebo (n=170). CT scans were performed every 8 weeks and quality of life (QoL) assessments every 4 weeks. Crossover to open-label TAS-102 was allowed. The primary endpoint of the TAGS study was overall survival (OS), which was already met and published [2]. Key secondary endpoints were PFS and QoL. Patient characteristics were similar in the treatment arms; the primary cancer site was gastric in 71% of patients in both arms and 44% of patients per arm had received prior gastrectomy. Approximately 37% of patients in each group had received 2 prior treatments, and about 63% had received 3 or more prior treatments, including fluoropyrimidine, platinum, irinotecan, taxanes, ramucirumab, and immunotherapy.
Results showed a superior survival benefit with FTD/TPI in patients treated in the third- and later-line, with a median OS of 6.8 months in the FTD/TPI arm versus 2.8 months in the placebo arm (HR 0.67; 95% CI 0.47-0.97; P=0.032, see Figure). There was also a higher PFS benefit in the FTD/TPI arm for third-line (HR 0.54; P=0.004) and fourth- or later-line (HR 0.57; P<0.001). Time to deterioration to ECOG ≥2 was also superior in the FTD/TPI arm for third-line (HR 0.60; P=0.005) and fourth- or later-line (HR 0.75; P=0.03). QoL was consistent in the third- and later-line with the overall population.
Figure: Median overall survival in the ITT population and 3L and 4L + subgroups [1]
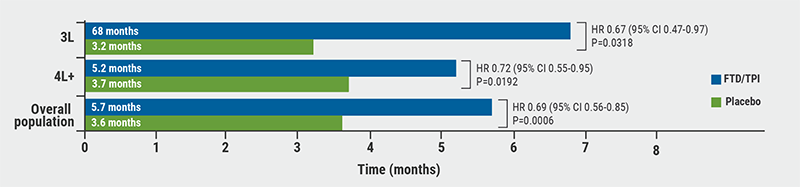
In short, this exploratory subgroup analysis of the TAGS study confirms the efficacy of FTD/TPI versus placebo for third- and later-line treatment of metastatic gastric cancer.
- Tabernero J, et al. Trifluridine/tipiracil outcomes in third- or later lines versus placebo in metastatic gastric cancer treatment: An exploratory subgroup analyses from the TAGS study. ASCO Gastrointestinal Cancers Symposium 2021, 15-17 January. Abstract 229.
- Shitara K, et al. Lancet Oncol. 2018 Nov;19(11):1437-1448.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Prognostic value of tumour deposits in stage 3 colon cancer patients Next Article
Neoadjuvant pembrolizumab in locally-advanced rectal cancer: primary results »
« Prognostic value of tumour deposits in stage 3 colon cancer patients Next Article
Neoadjuvant pembrolizumab in locally-advanced rectal cancer: primary results »
Related Articles
October 23, 2019
Ramosetron relieves low anterior resection syndrome
September 17, 2020
REGOMUNE: a phase 2 study combining regorafenib and avelumab
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

