https://doi.org/10.55788/0b543d83
Despite much progress in first-line treatment of metastatic urothelial cancer, improved second-line treatment remains an unmet clinical need [1]. Selective FGFR inhibition is becoming an increasingly important focus of novel drug development for this population [2]. Erdafitinib is a US-approved oral pan-FGFR tyrosine kinase inhibitor to treat locally advanced/metastatic urothelial carcinoma in patients with susceptible FGFR3/2 alterations who progressed after platinum-containing chemotherapy. Erdafitinib's accelerated approval was based on outcomes from a phase 2, single-arm trial [3].
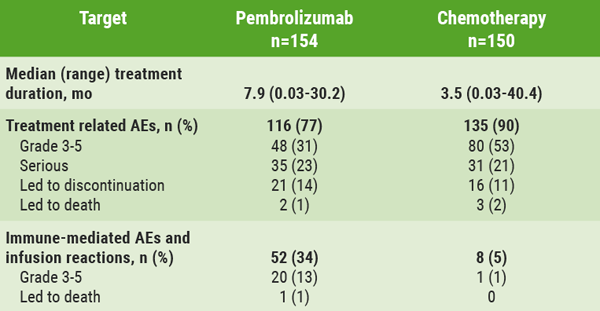
The recent phase 3, randomised THOR trial (NCT03390504) investigates the efficacy of erdafitinib as a second-line treatment versus standard of care in participants with unresectable, advanced or metastatic urothelial cancer. Results from Cohort 1 showed that erdafitinib significantly improved progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) versus chemotherapy in participants with an FGFR alteration [4]. In the current Cohort 2, erdafitinib was compared with pembrolizumab in the same participant group. Prof. Arlene Siefker-Radtke (MD Anderson Cancer Centre, TX, USA) presented the results [5].
Cohort 2 enrolled 351 (checkpoint inhibitor-naïve) participants who had progressed on first-line treatment. Participants were randomised 1:1 to erdafitinib or pembrolizumab. The primary endpoint was OS.
“In contrast to the results from Cohort 1, the trial did not meet its primary endpoint for Cohort 2,” summarised Prof. Siefker-Radtke. Neither the median OS nor the median PFS were statistically different between the groups. She continued: “This was somewhat unexpected because FGFR-altered tumours are known as ‘cold’ tumours, i.e. unresponsive for immune therapy. In line with this, we observed a significantly lower ORR in participants treated with pembrolizumab. However, the duration of response was longer in the pembrolizumab arm than in the erdafitinib arm.”
- Zheng X, et al. Front Oncol. 2022;12:907377.
- Garje R, et al. Oncologist. 2020;25(11):e1711–e1719.
- Loriot Y, et al. N Engl J Med. 2019;381:338–348.
- Loriot Y, et al. J Clin Oncol. 2023;41(17_suppl):LBA4619–LBA4619.
- Siefker-Radtke AO, et al. Phase III THOR study: Results of erdafitinib (erda) vs pembrolizumab (pembro) in pretreated patients (pts) with advanced or metastatic urothelial cancer (muc) with select fibroblast growth factor receptor alterations (FGFRalt). Abstract 2359O, ESMO 2023, 20–24 October, Madrid, Spain.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Lifileucel induces a durable response in heavily pretreated mucosal melanoma Next Article
LuPSMA and enzalutamide: a promising combination »
« Lifileucel induces a durable response in heavily pretreated mucosal melanoma Next Article
LuPSMA and enzalutamide: a promising combination »
Table of Contents: ESMO 2023
Featured articles
The importance of detecting early NSCLC
Breast Cancer
Benefit of pembrolizumab in TNBC remains after 5 years of follow-up
Addition of pembrolizumab promising in early-stage high-risk ER+/HER2- breast cancer
Long-term air pollution exposure at both residential and workplace locations increases breast cancer risk
Third-line datopotamab deruxtecan improves progression-free survival in previously treated metastatic HR+/HER2- breast cancer compared with chemotherapy
Colorectal Cancer
Neoadjuvant nivolumab/relatlimab demonstrates 100% pathological response in MMRd colon cancer
Selective KRASG12C inhibitor sotorasib leads to superior PFS in colorectal cancer
Postoperative ctDNA predicts survival in colorectal cancer
Overall survival in patients with initially unresectable colorectal liver metastases does not depend on choice of induction regimen
Lung Cancer
Perioperative nivolumab boosts event-free survival in NSCLC
Selective RET inhibitor selpercatinib doubles progression-free survival in RET-mutated NSCLC
Dato-DXd outperforms docetaxel in previously treated patients with metastatic NSCLC
First-line and second-line benefit of amivantamab in advanced, EGFR-mutated NSCLC
Upper Gastro-Intestinal Cancer
Perioperative durvalumab/FLOT improves pCR in gastric cancer
Active surveillance after neoadjuvant chemoradiotherapy in oesophageal cancer
FOLFIRINOX equals gemcitabine-based chemoradiotherapy in neoadjuvant setting for pancreatic cancer
Modified FLOT regime outperforms FOLFOX in advanced/metastatic gastric/gastroesophageal junction adenocarcinoma
Melanoma
Lifileucel induces a durable response in heavily pretreated mucosal melanoma
Darovasertib/crizotinib combination: a potential first-line therapy in metastatic uveal melanoma
Genito-Urinary Cancers
Two potential new first-line standards of care in metastatic urothelial cancer
LuPSMA and enzalutamide: a promising combination
No benefit of erdafitinib over pembrolizumab in urothelial cancer second-line therapy
Gynaecological Cancers
Addition of atezolizumab to chemotherapy and maintenance PARP inhibitor has no benefit in ovarian cancer
Short-induction chemotherapy improves survival in advanced cervical cancer
Neoadjuvant immune checkpoint blockade safe and effective in MMRd endometrial cancer
Featured Interviews
Can radiotracers predict response to PD-L1 inhibitors in early NSCLC?
The importance of detecting early NSCLC
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com