https://doi.org/10.55788/7a76a2bd
Claudin 18.2 is a tight-junction molecule predominantly found in the non-malignant gastric epithelium that becomes accessible on the tumour cell surface during malignant transformation, providing an appealing target for cancer therapy [1]. IBI343 is an ADC targeting claudin 18.2-expressing tumour cells. After claudin 18.2-dependent internalisation, the payload (exatecan, a topoisomerase inhibitor) induces apoptosis of the tumour cells. The released drug can also diffuse across the plasma membrane to reach and kill the neighbouring cells, resulting in a "bystander killing effect".
A phase 1 trial (NCT05458219) evaluated the safety and efficacy of IBI343 in 159 patients with heavily pretreated advanced G/GEJ cancer with confirmed claudin 18.2 expression. Dr Jia Liu (St Vincent’s Hospital Sydney, Australia) presented the results [2].
Safety findings showed that grade ≥3 treatment-related adverse events (AEs) occurred in 37.7% of the participants treated with 6 or 8 mg/kg IBI343. Gastrointestinal AEs of grade ≥3 occurred at low rates: vomiting 1.9%; nausea 1.3%; decreased appetite 1.3%. Hypoalbuminemia occurred in 24.5% of the participants and was of grade 1–2; no cases of interstitial lung disease (ILD) were reported.
In participants with moderate-to-high (≥40%) claudin 18.2 expression, the overall response rate was 31.2% and 41.4% in the 6 and 8 mg/kg IBI343 groups, respectively, with an associated disease control rate of 89.6% and 82.8% (see Figure). No response was observed in participants with low (<40%) claudin 18.2 expression.
Figure: Efficacy with IBI343 in G/GEJ cancer patients with moderate-to-high claudin 18.2 expression [2]
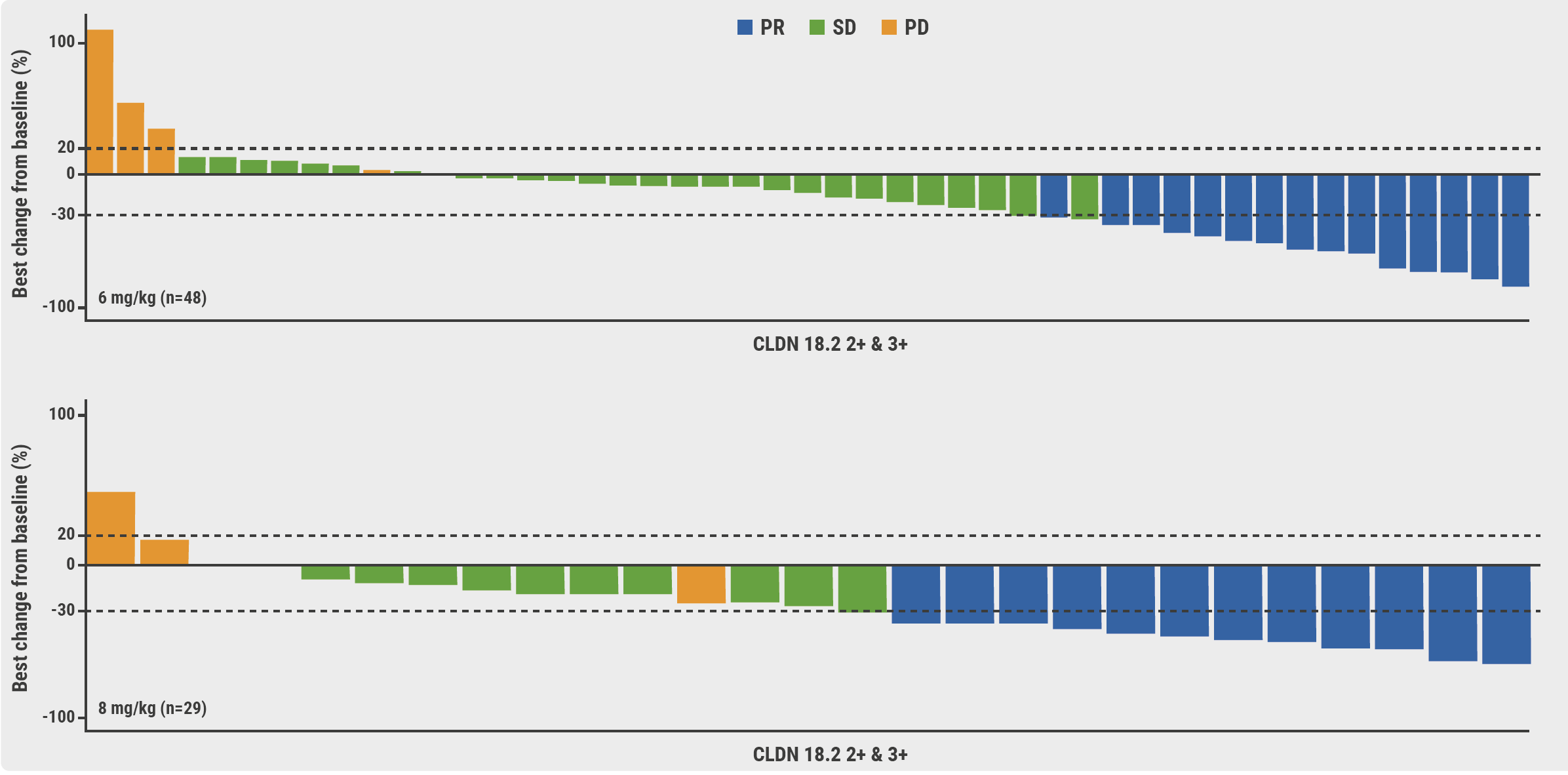
PD, progressive disease; PR, partial response; SD, stable disease.
Based on these results, Dr Liu concluded that “IBI343 is well-tolerated and has an encouraging efficacy in patients with G/GEJ cancer with moderate or high claudin 18.2 expression.” A registrational phase 3 clinical trial (NCT06238843) is being initiated.
- Nakayama I, et al. Nat Rev Clin Oncol. 2024;21:354-369.
- Liu JJ, et al. Anti-claudin 18.2 (CLDN18.2) antibody-drug conjugate (ADC) IBI343 in patients (pts) with solid tumors and gastric/gastro-esophageal junction adenocarcinoma (G/GEJ AC): A phase 1 study. Abstract 396MO, ESMO Gastrointestinal Cancers Congress 2024, 26–29 June, Munich, Germany.
Copyright ©2024 Medicom Medical Publishers
Posted on
Previous Article
« 177Lu-DOTATATE significantly extends PFS in patients with GEP-NETs, regardless of grade or origin Next Article
AI facilitates early detection of hepatocellular carcinoma »
« 177Lu-DOTATATE significantly extends PFS in patients with GEP-NETs, regardless of grade or origin Next Article
AI facilitates early detection of hepatocellular carcinoma »
Table of Contents: ESMO GI 2024
Featured articles
Gastric and Oesophageal Cancer
OS benefit in ARMANI, but is it worth it?
SPOTLIGHT on new targets in immunotherapy: claudin 18.2
Encouraging efficacy of anti-claudin 18.2 ADC in G/GEJ cancer
New analyses validate TAP and CPS scores for PD-L1 expression
KEYNOTE-585: negative trial, but long-term benefit in PD-L1-high/MSI subgroups?
Cancers of the Pancreas, Small Bowel, and Hepatobiliary Tract
AI facilitates early detection of hepatocellular carcinoma
177Lu-DOTATATE significantly extends PFS in patients with GEP-NETs, regardless of grade or origin
Durvalumab plus chemotherapy enhances 3-year survival in advanced biliary tract cancer
Promising first results of mitazalimab in metastatic pancreatic ductal adenocarcinoma
Cancers of the Colon, Rectum, and Anus
Post-operative MRD status more prognostic than TNM stage
CAPRI 2 GOIM trial navigates biomarker-driven therapy
Meta-analysis of triplet therapy in BRAFV600E-mutated mCRC
CheckMate 8HW: Nivolumab/ipilimumab in MSI-H/dMMR mCRC
Sequence effect for third-line treatment of mCRC
REGINA meets stage 1 endpoint in rectal cancer and moves to stage 2 with reduced dose regorafenib
High efficacy of pembrolizumab combined with standard therapy in patients with MSS/pMMR mCRC and high immune infiltrate
Prognostic value of ctDNA in stage III colon cancer
Neoadjuvant combined immunotherapy also effective in MSS/pMRR CRC
General GI Cancer
Peri- or post-operative chemotherapy benefits patients with resectable CRCLM
MINOTAUR: Promising phase 1 data for lunresertib plus FOLFIRI
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

