"The results from the VISIBLE 2 study confirm the efficacy and safety of vedolizumab subcutaneous (SC) maintenance therapy in patients with Crohn's disease (CD) who achieved a clinical response to vedolizumab intravenous induction therapy," lead study author Dr. Severine Vermeire of University Hospitals Leuven, in Belgium, told Reuters Health by email.
"Vedolizumab, the first gut-selective biologic for the treatment of inflammatory bowel disease, now offers IV and SC routes of administration for maintenance therapy," she added. "This greater choice in treatment formulation is in line with differing patient needs, preferences, and lifestyles."
With funding from Takeda, Dr. Vermeire and her colleagues enrolled adults who had moderate to severe active CD for least three months in the 3.5-year double-blind study at 169 sites in 30 countries. The participants also had inadequate response to or intolerance of corticosteroids, immunomodulators, and/or anti-tumor necrosis factor (TNF) therapies.
More than 600 patients received two intravenous doses of open-label vedolizumab 300 mg at weeks 0 and 2. The 410 patients who, at week 6, showed at least a 70-point decline in Crohn's Disease Activity Index (CDAI) score from baseline were randomized into two groups: 275 participants received maintenance vedolizumab 108 mg SC, and 135 patients received placebo, every two weeks through week 50.
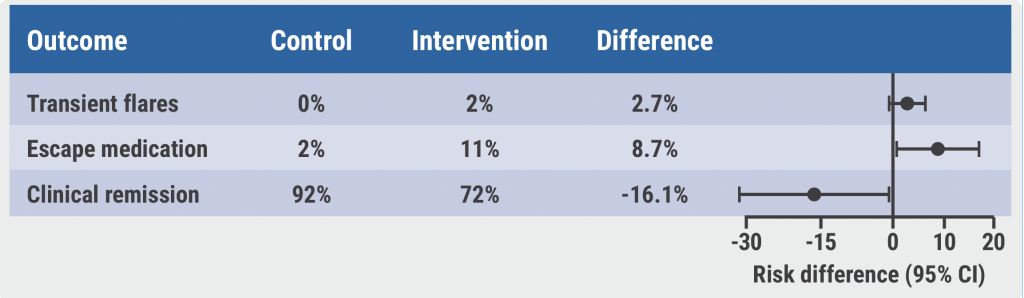
As reported in the Journal of Crohn's and Colitis, at week 52, 48% of patients receiving vedolizumab SC were in clinical remission, compared to 34% of those taking placebo (P=0.008).
But the drug's enhanced clinical response rate - a 100-point or greater improvement on the CDAI - was reached by 52% patients in the treatment arm versus 45% of those receiving placebo, a non-significant difference (P=0.167).
Also at week 52, 45% of patients taking vedolizumab and 18% of the placebo group were in corticosteroid-free clinical remission; and 49% of anti-TNF-naive patients on vedolizumab SC and 43% on placebo were in clinical remission.
Vedolizumab SC was well tolerated and safe, except for injection site reactions in eight (3%) participants.
Two independent experts who were not involved in the study welcomed the findings.
Dr. Mitra Barahimi, an assistant professor of gastroenterology at the University of Washington in Seattle, told Reuters Health by email, "While the study did not assess endoscopic outcomes, it did meet its primary endpoint of clinical remission and most of its secondary endpoints, importantly including steroid-free clinical remission, where a significant numerical difference of 27.1% was observed in favor of vedolizumab SC."
Dr. Brindusa Truta, an assistant professor of medicine at Johns Hopkins Medicine in Baltimore, Maryland, called the study "well-designed, with adequate sample population, conducted by highly experienced scientists."
"Subcutaneous vedolizumab formulation is important because it offers a new delivery method for inflammatory bowel disease patients with Crohn's disease who prefer to control where and when they receive their treatment," she told Reuters Health by email.
"Higher placebo rate may influence the lack of significance in clinical response and anti-TNF patients' clinical remission rates," Dr. Truta noted. "As specified by the authors, the expectation bias and the use of corticosteroids may be responsible for the high placebo rates."
"It would have been useful to include an intravenous vedolizumab reference arm in addition to a placebo arm to evaluate the difference in performance of the two formulations," she suggested.
All but one of the authors have financial relationships with Takeda, including employment.
SOURCE: https://bit.ly/3tpxTO2 Journal of Crohn's and Colitis, online August 17, 2021.
By Lorraine L. Janeczko
Posted on
Previous Article
« Electronic-nose technology promising for early detection of lung-allograft failure Next Article
Elevated lipoprotein(a) plus high sensitivity CRP levels raise cardiac risk »
« Electronic-nose technology promising for early detection of lung-allograft failure Next Article
Elevated lipoprotein(a) plus high sensitivity CRP levels raise cardiac risk »
Related Articles
April 12, 2022
Lessons from the COVID-19 pandemic for IBD management
August 18, 2021
Certain antibiotics may drive anti-TNF immunogenicity in IBD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

