Isocitrate dehydrogenase 1 (IDH1) is a key enzyme in cellular metabolism, epigenetic regulation, redox states, and DNA repair. IDH1 mutations are causal in the development and/or progression of various types of cancer due to the supraphysiological production of D-2-hydroxyglutarate (2HG). IDH1 mutations occur in 6–10% of AML patients [1]. Olutasidenib is a highly potent selective and orally active inhibitor of IDH1 mutants, inhibiting 2HG over-production.
The phase 2, multicohort 2102M 101 trial (NCT02719574) is evaluating olutasidenib as a single agent and in combination with azacitidine in IDH1 mutant AML patients. Dr Stéphane de Botton (Institute Gustave Roussy, France) presented interim results from cohort 1 in which 153 patients with relapsed or refractory AML received olutasidenib alone at the dose of 150 milligrams twice daily over continuous 28-day cycles [2]. The primary endpoint was complete remission (CR) plus complete remission with partial haematological recovery (CRh). Additional endpoints included overall response rate, duration of response, overall survival, transfusion independence, and safety.
At a median duration of treatment of 5.5 months, a total of 123 patients were evaluable for efficacy. The CR+CRh rate was 33%, including 30% of patients achieving CR. Composite CR (CR+CRh+CR with incomplete recovery) was 45%. The median duration of CR+CRh was not reached. The median duration of overall response was 11.7 months. The median overall survival for CR+CRh patients was not reached. For non-CR+CRh patients, the median overall survival was 15.0 months, for non-responders it was 4.1 months. Transfusion independence was achieved in all response groups, particularly those achieving CR.
Serious adverse events were reported in the majority of patients; the most frequent being febrile neutropenia, anaemia, and thrombocytopenia. Treatment-related adverse events of special interest included differentiation syndrome, which was reported in 21 patients (14%).
- Molenaar RJ, et al. Oncogene 2018;37:1949–1960.
- De Botton S, et al. Effect of olutasidenib (FT-2102) on complete remissions in patients with relapsed/refractory (R/R) mIDH1 acute myeloid leukemia (AML): Results from a planned interim analysis of a phase 2 clinical trial. Abstract 7006, ASCO 2021 Virtual Meeting, 4–8 June.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« VISION trial shows improved survival with 177Lu-PSMA-617 in mCRPC Next Article
Adjuvant chemotherapy does not improve outcome in patients with locally advanced cervical cancer »
« VISION trial shows improved survival with 177Lu-PSMA-617 in mCRPC Next Article
Adjuvant chemotherapy does not improve outcome in patients with locally advanced cervical cancer »
Table of Contents: ASCO 2021
Featured articles
Downloadable 1-Page Editor-Selected Trial PowerPoint Slides
Breast Cancer
Excellent prognosis for breast cancer patients with ultra-low-risk gene signature
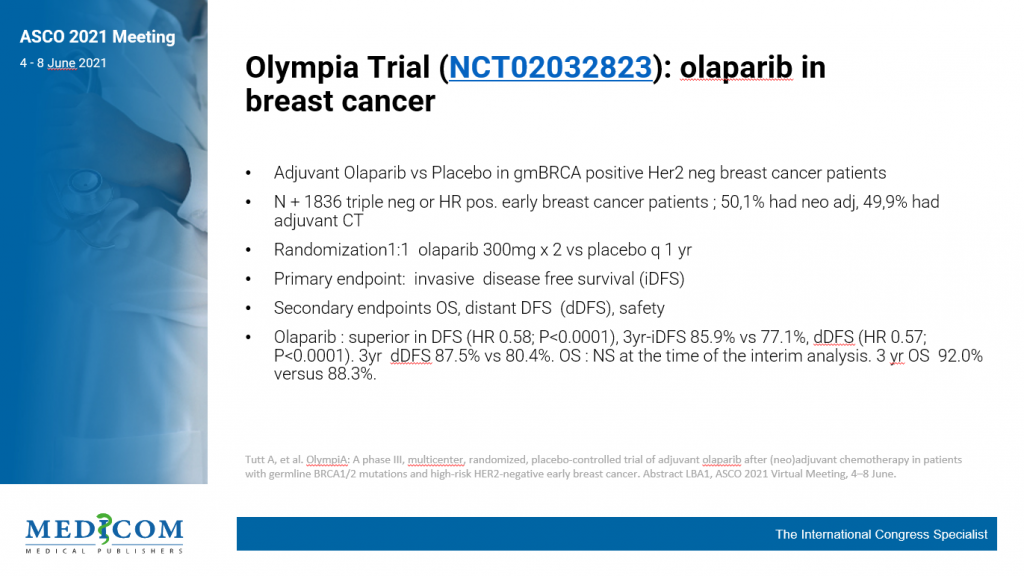
Olaparib benefits early breast cancer patients with BRCA1/2 germline mutation
Platinum-based adjuvant chemotherapy in TNBC is not superior or non-inferior to capecitabine
Dalpiciclib benefits patients with HR-positive, HER2-negative advanced breast cancer
Trastuzumab-deruxtecan showed clinical activity in patients with brain metastases
Lung Cancer
Neoadjuvant nivolumab plus chemotherapy improves surgical outcomes in NSCLC
Immune-related adverse events are associated with efficacy of atezolizumab in patients with advanced NSCLC
Sustained efficacy of nivolumab/ipilimumab plus 2 cycles of chemotherapy in NSCLC
Patritumab deruxtecan (HER3-DXd) in EGFR TKI-resistant NSCLC
Melanoma
Long-term results from ground-breaking melanoma trials
Novel dual checkpoint blockade improves progression-free survival in melanoma
Neoadjuvant therapy with nivolumab plus relatlimab is safe and effective in patients with stage III melanoma
Genitourinary Cancers
VISION trial shows improved survival with 177Lu-PSMA-617 in mCRPC
Abiraterone added to ADT + docetaxel nearly doubles survival in de novo mCSPC
Post-nephrectomy pembrolizumab improves disease-free survival
Glutaminase inhibitor telaglenastat does not improve survival mRCC
Promising efficacy and safety of feladilimab in recurrent/metastatic urothelial carcinoma
Gastrointestinal Cancers
Pembrolizumab benefits survival in MSI-H/dMMR metastastic colorectal cancer
Panitumumab added to 5-FU/LV effective as maintenance therapy in patients with mCRC
Trastuzumab-deruxtecan showed promising activity in patients with HER2-expressing mCRC
Benefit of both I-O/chemo combo and I-O/I-O combo over chemotherapy alone in oesophageal squamous cell cancer
Benefit of I-O/chemo combo over chemotherapy alone in advanced GC/GEJC/EAC
Perioperative chemotherapy and neoadjuvant multimodality therapy appear equally effective
Haematological Cancers
Olutasidenib demonstrates efficacy in patients with relapsed/refractory IDH1 mutant AML
Acalabrutinib as effective but better tolerated than ibrutinib in CLL
Gynaecological Cancers
Adjuvant chemotherapy does not improve outcome in patients with locally advanced cervical cancer
Novel drug combination for recurrent ovarian cancer
Dual HER2-blockade shows anti-tumour activity in patients with uterine cancer
Paediatric Cancer
Molecular tumour profiling impacts the diagnosis and treatment of solid tumours
Circulating tumour DNA to evaluate response in children with neuroblastoma
Basic Science
PARP7 inhibitor shows promising results in first-in-human trial
IACS-6274 is well tolerated and biologically active in selected advanced tumours
CYT-0851 shows promising anti-tumour activity across different tumour types
Related Articles
August 12, 2021
Long-term results from ground-breaking melanoma trials
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

