TYK2 is an intracellular kinase that mediates IL-23, IL-12, and type 1 interferon signalling in the pathogenesis of psoriasis. Deucravacitinib has a unique mechanism of action distinct from JAK1/2/3 inhibitors. It achieves a high degree of selectivity by uniquely binding to the TYK2 regulatory domain, rather than to the active domain of TYK2, which is structurally distinct from the regulatory domains of JAK1/2/3 [2]. Deucravacitinib previously demonstrated efficacy and tolerability in phase 2 trials in patients with moderate-to-severe plaque psoriasis and active psoriatic arthritis [3,4].
POETYK PSO-1 (NCT03624127) and PSO-2 (NCT03611751) were two phase 3, double-blind, 52-week trials that randomised patients with moderate-to-severe plaque psoriasis (BSA ≥10%, PASI ≥12, sPGA ≥3) to placebo, deucravacitinib 6 mg once daily, or apremilast 30 mg twice daily. POETYK PSO-1 included 666 patients and POETYK PSO-2 included 1,020 patients [1].
The co-primary endpoints, PASI 75 and sPGA 0/1 response versus placebo at week 16, were achieved in both trials. In addition, statistical significance was met for deucravacitinib versus placebo and apremilast for multiple ranked secondary endpoints. Significantly greater proportions of patients in the deucravacitinib versus placebo and apremilast arms achieved PASI 75 and sPGA 0/1 responses at week 16. Deucravacitinib responses increased beyond week 16 and were also superior to apremilast at week 24 in both trials (P<0.0001 for all comparisons; see Figure).
Figure: PASI 75 response rates of POETYK PSO-1 and POETYK PSO-2 [1]
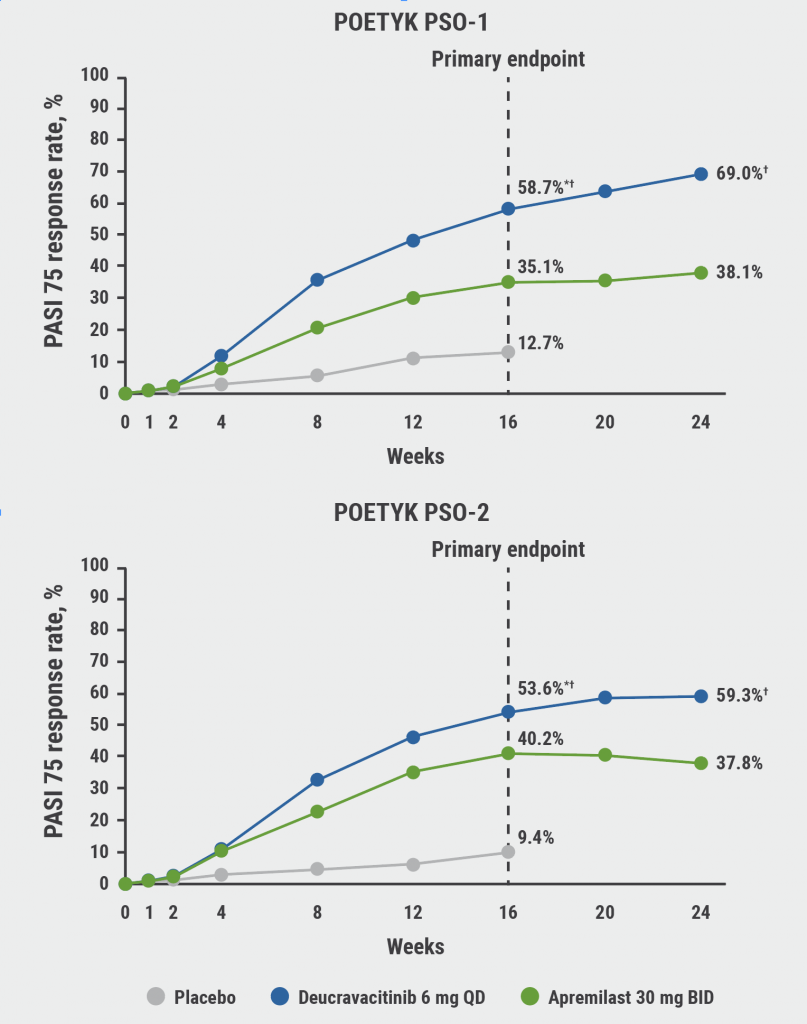
*P<0.0001 vs placebo; ƗP<0.0001 vs apremilast.
PASI, Psoriasis Area Severity Index; QD, once daily; BID, twice daily.
During the 16-week, placebo-controlled periods, the most common adverse events (AEs) were nasopharyngitis, upper respiratory tract infections, headache, diarrhoea, and nausea. Overall AEs, serious AEs, and AEs leading to discontinuation were similar across the 3 groups. No clinically meaningful changes were observed in laboratory parameters during the 2 trials. Deucravacitinib was well tolerated and had a similar safety profile in both trials.
Based on these findings deucravacitinib has the potential to become an efficacious and well-tolerated treatment of choice for patients with moderate-to-severe plaque psoriasis.
- Armstrong A. Deucravacitinib, an Oral, Selective Tyrosine Kinase 2 (TYK2) Inhibitor, Compared With Placebo and Apremilast in Moderate to Severe Plaque Psoriasis: Efficacy and Safety Results From the Phase 3 POETYK PSO-1 and POETYK PSO-2 Trials. Abstract O6, 6thWorld Psoriasis & Psoriatic Arthritis Conference, 30 June–3 July 2021.
- Burke JR, et al. Sci Transl Med. 2019;11:eaaw1736.
- Papp K, et al. N Engl J Med. 2018;379:1313–1321.
- Mease PJ, et al. ACR 2020, 5–9 November.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Blacks with hypertension also seem to benefit from amlodipine with benazepril Next Article
Rapid pustule and skin clearance with IL-36 receptor inhibitor spesolimab »
« Blacks with hypertension also seem to benefit from amlodipine with benazepril Next Article
Rapid pustule and skin clearance with IL-36 receptor inhibitor spesolimab »
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

