https://doi.org/10.55788/b8ba085d
“Alvelestat is an oral, selective neutrophil elastase inhibitor, which may have some advantages over plasma-derived AAT, the current standard-of-care for patients with AATD-LD,” mentioned Prof. Robert Stockley (University Hospital Birmingham, UK) at the start of his presentation. “It results in effective lung penetration and is resistant to oxidative inactivation,” he clarified. The phase 2 ASTRAEUS study (NCT03636347) aimed to evaluate the effect of alvelestat on biomarkers of AATD-LD over 12 weeks [1]. The included patients were randomised to 1 of 2 doses of alvelestat, 120 mg, twice daily (n=22); 240 mg, twice daily (n=40), or to a placebo (n=36).
After 12 weeks of treatment, the change from baseline blood neutrophil elastase activity was significantly larger in the experimental groups than in the placebo group, displayed as the percentage change in the least squared means (LSM):
- Placebo: -18.1%
- Alvelestat 120 mg: -83.5%; P=0.001
- Alvelestat 240 mg: -93.3%; P<0.001
- Stockley RA, et al. Alvelestat, an Oral Neutrophil Elastase Inhibitor in Alpha-1 Antitrypsin Deficiency (AATD): Results of a Phase II Trial. C15, ATS International Conference 2023, 19–24 May, Washington DC, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Fewer exacerbations with dupilumab in COPD with type 2 inflammation Next Article
Practice-changing results for ensifentrine in COPD »
« Fewer exacerbations with dupilumab in COPD with type 2 inflammation Next Article
Practice-changing results for ensifentrine in COPD »
Table of Contents: ATS 2023
Featured articles
TORREY trial: seralutinib associated with reduced pulmonary vascular resistance in PAH
Practice-changing results for ensifentrine in COPD
Asthma
Vitamin D for asthma protection: what is the optimal timing?
Remarkable results for novel biologic therapy for asthma
Success for fluticasone furoate plus vilanterol in uncontrolled asthma
COPD
Practice-changing results for ensifentrine in COPD
Alvelestat for AATD meets primary endpoints in phase 2 trial
Fewer exacerbations with dupilumab in COPD with type 2 inflammation
New AATD therapy may alleviate treatment burden for patients
Efficient detection of AECOPD with at-home vital signs monitoring
COVID-19
COVA trial: success for the investigational agent sarconeos in hospitalised COVID-19
Mitochondrial DNA levels predict COVID-19 severity
Other
Excellent results for C21 in idiopathic pulmonary fibrosis
Can camlipixant improve quality of life of patients with refractory chronic cough?
TORREY trial: seralutinib associated with reduced pulmonary vascular resistance in PAH
Improving quality care in sepsis through machine learning models
Can information technology improve guideline adherence in sepsis care?
Related Articles
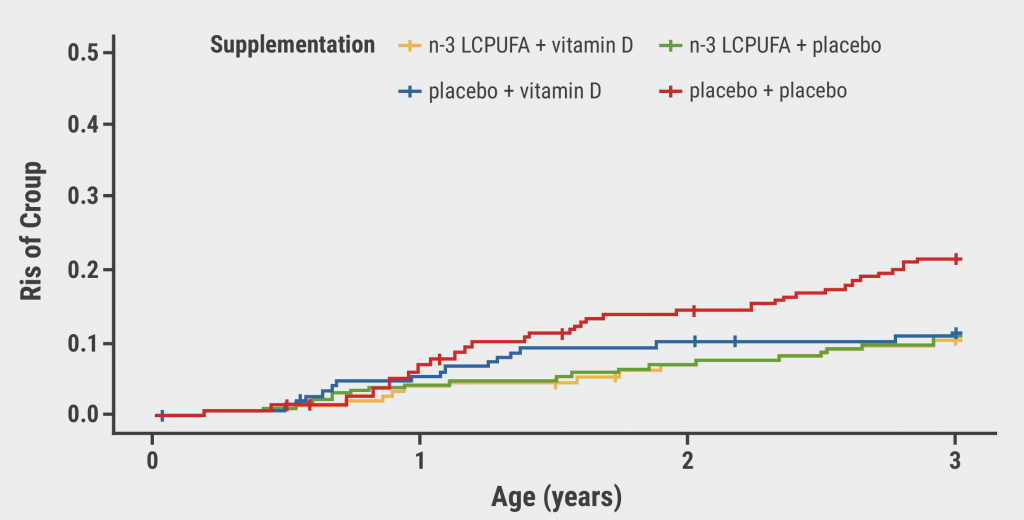
October 30, 2022
Fish oil or vitamin D during pregnancy can prevent croup
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

