https://doi.org/10.55788/0c4ba87f
“The treatment options for patients with pre-treated AGOC are limited,” said Prof. Nick Pavlakis (University of Sydney, Australia) at the start of his presentation [1]. “However, regorafenib showed promising anti-tumour activity in a phase 2 trial [2].” After these findings had been reported, a phase 3 study was designed to test regorafenib against placebo. The INTEGRATE IIa trial (NCT02773524) randomised 251 patients with AGOC who had received at least 2 prior lines of therapy 2:1 to regorafenib, 160 mg, once daily at days 1 to 21 of a 28-day cycle, or placebo [3]. The primary endpoint was the overall survival (OS).
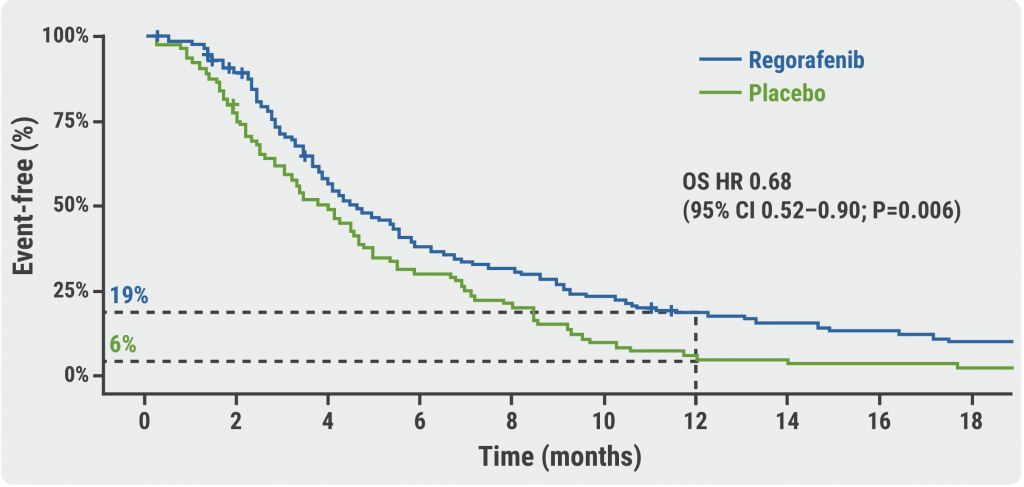
Patients in the regorafenib arm had an OS benefit compared with patients in the placebo arm (HR 0.68; 95% CI 0.52–0.90; P=0.006; see Figure) . The corresponding 12-month OS rates were 19% and 6%, respectively. Prof. Pavlakis mentioned that there were no significant differences among pre-defined subgroups. Furthermore, the progression-free survival results confirmed the OS results, demonstrating a benefit for patients on regorafenib over patients on placebo (HR 0.53; 95% CI 0.40–0.70; P<0.0001). Interestingly, patients receiving regorafenib displayed a significant delay in the deterioration of their global quality-of-life compared with patients receiving placebo (P=0.0043). According to Prof. Pavlakis, the safety profile of regorafenib was comparable with that of previously reported studies on this agent.
Figure: Overall survival of regorafenib versus placebo [3]
In conclusion, regorafenib improved the health outcomes in patients with pre-treated AGOC, presenting itself as a new treatment option for the population. The agent is further assessed in combination with nivolumab against chemotherapy in an estimated 450 patients with AGOC who failed on at least 2 prior lines of therapy in the INTEGRATE IIb trial (NCT04879368).
- Smyth EC, et al. Lancet. 2020;396:635–648.
- Pavlakis N, et al. JCO. 2016;34:2728–2735.
- Pavlakis N, et al. INTEGRATE IIa: A randomised, double-blind, phase III study of regorafenib versus placebo in refractory advanced gastro-oesophageal cancer (AGOC)—A study led by the Australasian Gastro-intestinal Trials Group (AGITG). Late-breaking abstract 294, ASCO GI 2023, 19–21 January, San Francisco, CA, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Radiotherapy or not in locally advanced oesophageal or junctional cancer? Next Article
Zolbetuximab plus mFOLFOX6 successful in CLDN18.2-positive subgroup of gastric cancer »
« Radiotherapy or not in locally advanced oesophageal or junctional cancer? Next Article
Zolbetuximab plus mFOLFOX6 successful in CLDN18.2-positive subgroup of gastric cancer »
Table of Contents: ASCO GI 2023
Featured articles
Oesophageal and Gastric Cancer
Zolbetuximab plus mFOLFOX6 successful in CLDN18.2-positive subgroup of gastric cancer
Regorafenib offers survival benefit for patients with pre-treated gastric cancer
Radiotherapy or not in locally advanced oesophageal or junctional cancer?
Neoadjuvant immunotherapy is safe and efficacious in a phase 2 gastric cancer trial
S-1 adjuvant chemotherapy: 4 or 8 courses in stage 2 gastric cancer?
LATG/LAPG demonstrates excellent long-term efficacy in stage 1 gastric cancer
3-year follow-up data confirms benefits of nivolumab plus chemotherapy
Long-term results for nivolumab plus chemotherapy and nivolumab plus ipilimumab in oesophageal cancer
Promising phase 2 results for HER-Vaxx in gastric cancer
Anal and Colorectal Cancer
IMbrave 151 missed primary endpoint in advanced BTC
Combination botensilimab plus balstilimab demonstrates promising activity in heavily pre-treated MSS CRC
Mutation-based selection to identify patients suitable for panitumumab treatment
Fucoidan associated with quality-of-life benefits in patients with rectal cancer receiving CCRT
ctDNA appears useful in monitoring patients with anal cancer undergoing CRT
SUNLIGHT trial meets primary endpoint in refractory metastatic CRC
Does cell-free DNA influence MRD testing in post-operative colon cancer?
OPERA: surgery may be avoided with adequate therapy in rectal cancer
Can we improve total neoadjuvant therapy for rectal cancer?
Hepatobiliary Cancer
Palliative radiation therapy improves hepatic pain in HCC and liver metastasis
Improved survival following postoperative sorafenib plus TACE in HCC
Quality-of-life benefits for tislelizumab in uHCC
Stereotactic body radiation therapy beneficial for patients with locally advanced HCC
SWOG 1815, first-ever phase 3 trial in BTC, fails
Acceptable safety profile and encouraging efficacy of nanvuranlat in BTC
Pancreatic Cancer
First-line NALIRIFOX superior to standard treatment in mPDAC
Novel approach delivers quality-of-life benefits for patients with pancreatic cancer
Related Articles
April 14, 2020
Increased risk of small bowel cancer in IBD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

