https://doi.org/10.55788/f37566dc
“The combination of the PD-L1 inhibitor atezolizumab and the VEGF inhibitor bevacizumab is the main standard-of-care for patients with unresectable hepatocellular carcinoma (HCC) [1],” explained Prof. Anthony El-Khoueiry (USC Norris Comprehensive Cancer Center, CA, USA). The phase 2 IMbrave151 study (NCT04677504) evaluated atezolizumab and bevacizumab in combination with chemotherapy in newly diagnosed patients with advanced BTC (n=162) [2].
Patients who were randomised to the experimental arm received 8 cycles of gemcitabine plus cisplatin chemotherapy and bevacizumab and atezolizumab until disease progression, unacceptable toxicity, or until there was no longer a clinical benefit. In the placebo arm, bevacizumab was replaced with placebo. PFS was the primary endpoint.
PFS was not significantly improved in the bevacizumab arm compared with the control arm, although the investigators noticed a numerical benefit of the experimental arm over the control arm (median PFS 8.3 months vs 7.9 months; HR 0.76; 95% CI 0.51–1.14, see Figure). The overall survival (OS) data displayed a similar trend (median OS ‘not reached’ vs 11.4 months; HR 0.74; 95% CI 0.43–1.27). Prof. El-Khoueiry mentioned that bevacizumab did not add substantial toxicity to the safety profile of the treatment regimen. The rates of grade 3 or 4 adverse events (AEs) during the chemotherapy phase were similar for the 2 treatment arms, with 69.2% and 70.4%. After the chemotherapy phase, these rates dropped to 31.5% in the bevacizumab arm and to 24.5% in the control arm. Finally, it was highlighted that any grade hypertension was more common in the bevacizumab arm than in the placebo arm (34.6% vs 16.0%).
Figure: Progression-free survival of bevacizumab-containing regimen versus placebo [2]
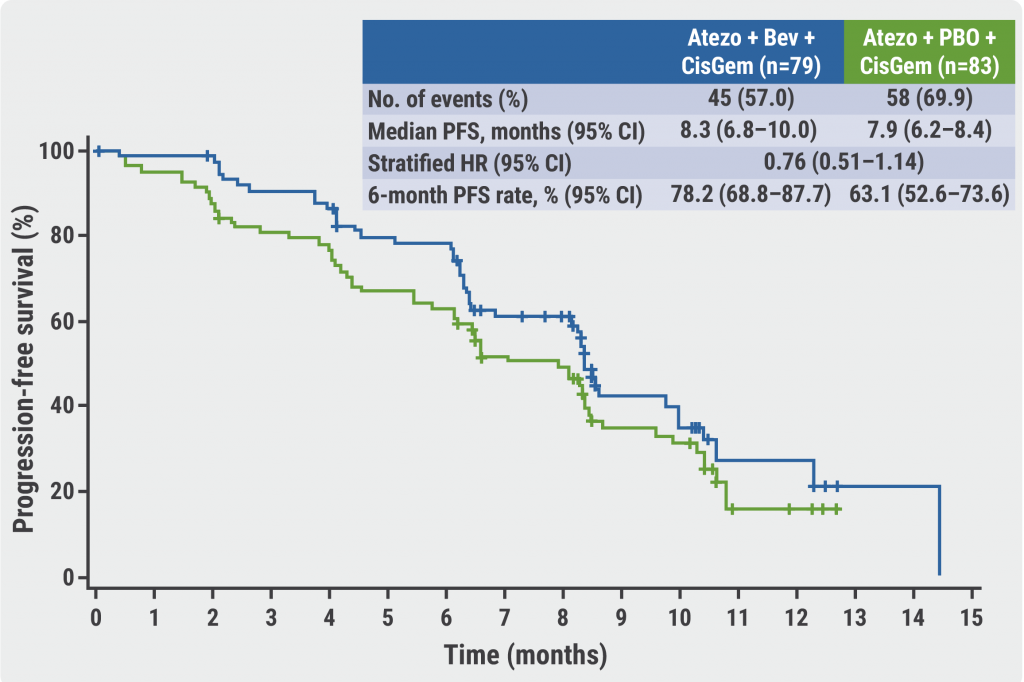
Atezo, atezolizumab; Bev, bevacizumab; CisGem, cisplatin and gemcitabine; PBO, placebo.
Although the results of the IMbrave 151 study showed a modest improvement in PFS with the addition of bevacizumab to atezolizumab and chemotherapy in patients with newly diagnosed advanced BTC, this finding did not reach statistical significance. The 2 arms were experimental with a small sample size.
- Finn RS, et al. N Engl J Med. 2020;382:1894–1905.
- El-Khoueiry AB, et al. IMbrave151: A phase 2, randomized, double-blind, placebo-controlled study of atezolizumab with or without bevacizumab in combination with cisplatin plus gemcitabine in patients with untreated, advanced biliary tract cancer. Abstract 491, ASCO GI 2023, 19–21 January, San Francisco, CA, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Acceptable safety profile and encouraging efficacy of nanvuranlat in BTC Next Article
SWOG 1815, first-ever phase 3 trial in BTC, fails »
« Acceptable safety profile and encouraging efficacy of nanvuranlat in BTC Next Article
SWOG 1815, first-ever phase 3 trial in BTC, fails »
Table of Contents: ASCO GI 2023
Featured articles
Oesophageal and Gastric Cancer
Zolbetuximab plus mFOLFOX6 successful in CLDN18.2-positive subgroup of gastric cancer
Regorafenib offers survival benefit for patients with pre-treated gastric cancer
Radiotherapy or not in locally advanced oesophageal or junctional cancer?
Neoadjuvant immunotherapy is safe and efficacious in a phase 2 gastric cancer trial
S-1 adjuvant chemotherapy: 4 or 8 courses in stage 2 gastric cancer?
LATG/LAPG demonstrates excellent long-term efficacy in stage 1 gastric cancer
3-year follow-up data confirms benefits of nivolumab plus chemotherapy
Long-term results for nivolumab plus chemotherapy and nivolumab plus ipilimumab in oesophageal cancer
Promising phase 2 results for HER-Vaxx in gastric cancer
Anal and Colorectal Cancer
IMbrave 151 missed primary endpoint in advanced BTC
Combination botensilimab plus balstilimab demonstrates promising activity in heavily pre-treated MSS CRC
Mutation-based selection to identify patients suitable for panitumumab treatment
Fucoidan associated with quality-of-life benefits in patients with rectal cancer receiving CCRT
ctDNA appears useful in monitoring patients with anal cancer undergoing CRT
SUNLIGHT trial meets primary endpoint in refractory metastatic CRC
Does cell-free DNA influence MRD testing in post-operative colon cancer?
OPERA: surgery may be avoided with adequate therapy in rectal cancer
Can we improve total neoadjuvant therapy for rectal cancer?
Hepatobiliary Cancer
Palliative radiation therapy improves hepatic pain in HCC and liver metastasis
Improved survival following postoperative sorafenib plus TACE in HCC
Quality-of-life benefits for tislelizumab in uHCC
Stereotactic body radiation therapy beneficial for patients with locally advanced HCC
SWOG 1815, first-ever phase 3 trial in BTC, fails
Acceptable safety profile and encouraging efficacy of nanvuranlat in BTC
Pancreatic Cancer
First-line NALIRIFOX superior to standard treatment in mPDAC
Novel approach delivers quality-of-life benefits for patients with pancreatic cancer
Related Articles
April 14, 2020
Increased incidence of colorectal cancer and death in CD
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

