The phase 3 KEYNOTE-590 trial (NCT03189719) was designed to assess whether pembrolizumab could offer additional benefits over chemotherapy alone to patients with oesophageal cancer and EGJ Siewert type 1. In total, 749 patients with locally advanced unresectable or metastatic oesophageal cancer (mixed histologies: adenocarcinoma and squamous cell carcinoma) were randomised 1:1 to chemotherapy plus pembrolizumab (200 mg, intravenous, every 3 weeks) or chemotherapy plus placebo. The primary analysis showed that additional pembrolizumab was associated with improved overall survival (OS; HR 0.73; P<0.001), progression-free survival (PFS; HR 0.65; P<0.001), and anti-tumour activity. Dr Jean-Philippe Metges (CHU Brest, France) presented the results after 12 months of additional follow-up.
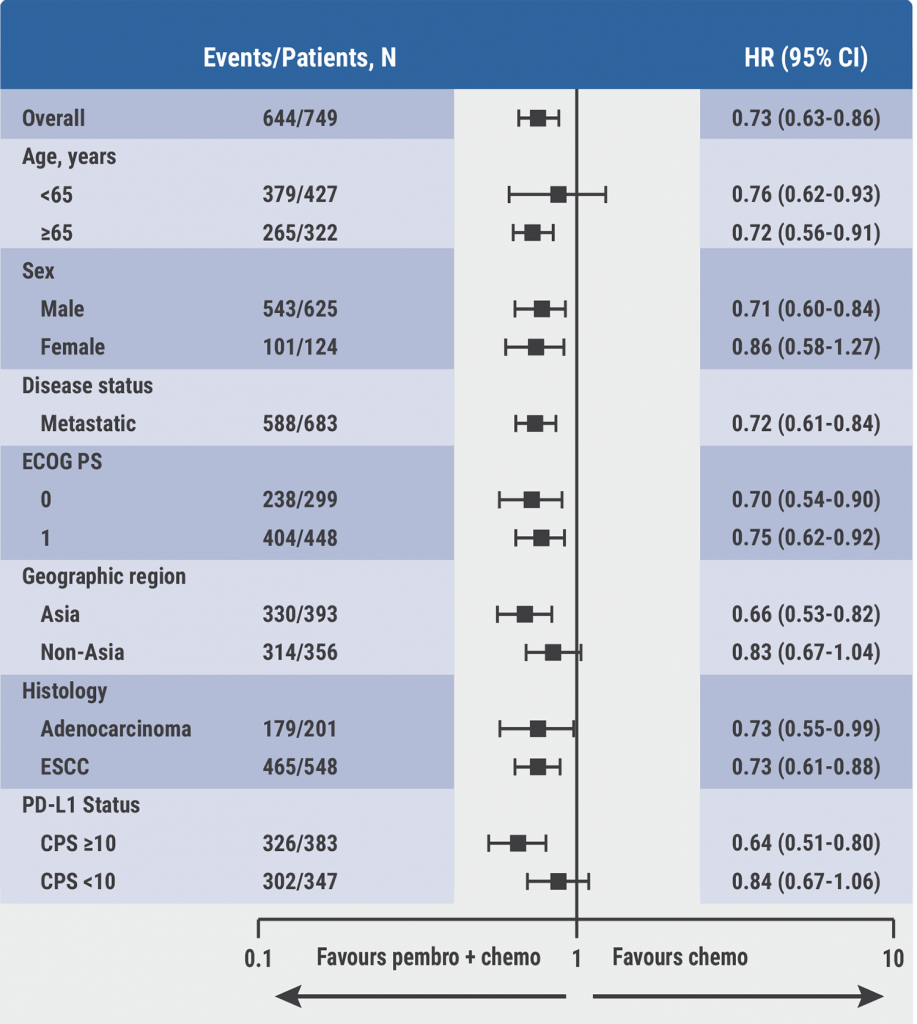
After a median follow-up of 34.8 months, the data showed that the benefits of pembrolizumab were maintained, with a median OS of 12.4 versus 9.8 months (HR 0.73; P<0.001) and a median PFS of 6.3 versus 5.8 months (HR 0.64; P<0.001). The 2-year OS rates were 26% and 16% for patients receiving pembrolizumab or placebo, respectively. In addition, the 2-year PFS rates were 12% and 3%. The results were consistent across pre-defined subgroups, including patients with adenocarcinoma (see Figure). Moreover, the anti-tumour response data showed that 20.4% of the patients on pembrolizumab had a response duration of 24 months or longer. In the placebo group, 6.2% of the patients had a response duration of a minimum of 2 years. Also, the quality of life for patients in the pembrolizumab or placebo arm was comparable.
Figure: Subgroup analyses of overall survival in KEYNOTE-590 [1]
Importantly, the addition of pembrolizumab was not associated with increased toxicity, according to Dr Metges. The treatment arms had similar safety profiles, with 71.9% and 67.7% of the patients experiencing grade 3 or higher adverse events (AEs) in the pembrolizumab arm and placebo arm, respectively. The rate of grade 3 immune-mediated AEs was higher in the pembrolizumab arm (7.0% vs 2.2%).
Dr Metges concluded that the longer-term follow-up data of the KEYNOTE-590 trial confirm that pembrolizumab plus chemotherapy is a new first-line standard-of-care option for patients with locally advanced or metastatic oesophageal cancer, including patients with adenocarcinoma. It is important to note that the best results were shown in patients with PD-L1 CPS ≥10 (HR 0.64).
Updated KEYNOTE-062 results
The KEYNOTE-062 trial (NCT02494583) randomised 763 patients to pembrolizumab alone, chemotherapy alone, or pembrolizumab plus chemotherapy. The primary analysis showed that the combination regimen did not outperform chemotherapy alone with respect to OS and PFS [3]. However, pembrolizumab alone displayed non-inferiority to chemotherapy. Dr Zev Wainberg (University College Los Angeles, CA, USA) presented the updated results after 25 months of additional follow-up [2].
Pembrolizumab displayed non-inferiority to chemotherapy alone in patients with CPS ≥1, with 24-month OS rates of 26.6% for the pembrolizumab arm versus 18.8% for the chemotherapy arm (HR 0.90). This result was more pronounced in patients with CPS ≥10 (24-months OS rates: 39.1% vs 21.1%; HR 0.62).
The combination regimen did not significantly outperform chemotherapy alone in this population. The 24-month OS rates for patients with CPS ≥1 were 24.5% in the combination arm and 18.8% in the chemotherapy arm. In patients with CPS ≥10, the corresponding results were 28.3% and 21.1%, respectively. According to Dr Wainberg, the updated safety profile was similar to that of the primary analysis. Grade 3-5 adverse events were less common in the pembrolizumab arm (50.4%) compared with the chemotherapy arm (82.4%) or the combination arm (84.8%).
The combination of pembrolizumab plus chemotherapy for patients with gastric adenocarcinoma or GEJAC is further assessed in the KEYNOTE-859 trial (NCT03675737).
- Metges J-P, et al. First-line Pembrolizumab Plus Chemotherapy Versus Chemotherapy in Advanced EsophagealCancer: Longer-term Efficacy, Safety, and Quality-of-life Results Fromthe Phase 3 KEYNOTE-590 Study. Abstract 241, ASCO GI 2022, 20–22 January.
- Wainberg ZA, et al. Pembrolizumab With or Without Chemotherapy Versus Chemotherapy Alone for Patients With PD-L1-Positive Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: Update From the Phase 3 KEYNOTE-062 Trial. Abstract 243, ASCO GI 2022, 20–22 January.
- Shitara K, et al. JAMA Oncol. 2020;6(10):1571–1580.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« NIPICOL: New data on optimal ICI treatment duration in MSI/dMMR mCRC Next Article
Nivolumab in gastric cancer: Efficacy update and the role of gut microbiome »
« NIPICOL: New data on optimal ICI treatment duration in MSI/dMMR mCRC Next Article
Nivolumab in gastric cancer: Efficacy update and the role of gut microbiome »
Related Articles
March 21, 2022
Key updates in colorectal cancer screening
March 21, 2022
EMR versus ESD in oesophageal cancer
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

