The study included 754 participants from the Mayo Clinic Study of Aging with genome-wide genotype and regional tau-PET (AV-1451) data. The mean age was 72.4 years, 54.6% were male, and 87.4% were cognitively impaired. A genome-wide association study of tau burden of the ERC (a sensitive marker of early tau deposition) was performed of 515,206 single nucleotide polymorphisms (SNPs) following genotyping with the Illumina GSA array. A post-hoc stratified analysis utilised amyloid-PET positivity (global PiB >1.48) as a discriminator.
A genome-wide significant association was found for rs75546066, in an intergenic region on chromosome 9. The minor allele (A, frequency 2.7%) was associated with lower ERC tau (P=2.85·10-8; β=-0.49). The effect was stronger in amyloid-negative versus amyloid-positive individuals (β=-0.51 and -0.23, respectively).
The observation that tau deposition may have a genetic architecture distinct from known AD risk genes could have implications for enhanced risk prediction, according to the authors, as well as for therapeutic targeting.
- Ramanan V, et al. Abstract S4.009, AAN 2020.
Posted on
Previous Article
« News on AD biomarkers Next Article
Non-Alzheimer’s disease pathophysiology in the elderly »
« News on AD biomarkers Next Article
Non-Alzheimer’s disease pathophysiology in the elderly »
Table of Contents: EAN 2020
Featured articles
Alzheimer's Disease and Other Dementias
Non-Alzheimer’s disease pathophysiology in the elderly
Novel genetic association with resistance to ERC tau deposition
Diastolic dysfunction novel risk factor for cognitive impairment
Epilepsy
Avoidable epilepsy-related mortality remains high
How genetic testing can contribute to epilepsy management
Cenobamate effective in focal epilepsy
Sustained seizure reductions with cannabidiol for Lennox-Gastaut syndrome
Prevalence of autoantibodies in epilepsy almost 10%
Parkinson's Disease
White matter matters in Parkinson’s disease
Sleep disorders mark PD progression
Directional DBS superior to omnidirectional DBS
Stroke
Benefits of statins to prevent stroke outweigh risks
Extubation after thrombectomy: the sooner, the better
Thrombus location and length predictors of early neurological deterioration
Endovascular treatment in large vessel occlusion stroke patients treated with OAC
Early edoxaban may be safe after cardioembolic stroke
Headache and Pain
Small fibre pathology as biomarker for fibromyalgia
Migraine as a cyclical functional disorder
Reassuring real-world safety profile of 3 CGRP inhibitors
Long-term cardiovascular safety of erenumab
Real-world data for erenumab in Germany
Eptinezumab in chronic migraine and medication-overuse headache
Fremanezumab tolerability in cardiovascular patients with migraine
Effects of galcanezumab on health-related quality of life
Multiple Sclerosis
Imaging to evaluate remyelination and neuroprotection
Serum NfL predicts long-term clinical outcomes in MS
Epstein-Barr virus-targeted T-cell immunotherapy for progressive MS
High NEDA rates after 2 years of ocrelizumab
Switching from natalizumab to moderate- versus high-efficacy DMT
Results of compounds in late stages of development
Alemtuzumab efficacy and safety data of over 9 years
Fampridine treatment results in routine clinical practice
Air pollution is a possible risk factor for MS
Neuromyelitis Optica Spectrum Disorder
Genetic association studies in NMOSD needed
Eculizumab in NMOSD: the PREVENT study
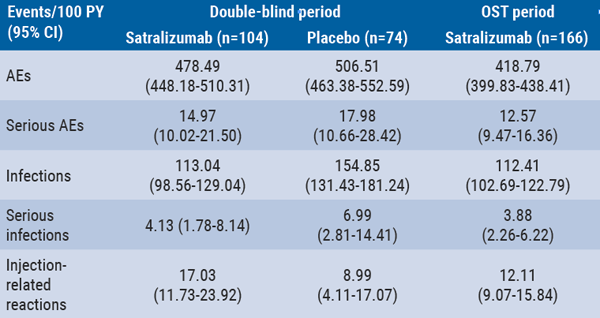
Long-term safety of satralizumab consistent with double-blind periods
Neuromuscular Disorders
Biomarkers predicting motor function in SMA
Sustained benefits of avalglucosidase alfa in late-onset Pompe disease
Efficacy and safety of rituximab in refractory MG corroborated
Related Articles
September 10, 2020
Small fibre pathology as biomarker for fibromyalgia
September 10, 2020
Long-term safety of satralizumab consistent with double-blind periods
September 10, 2020
Epstein-Barr virus-targeted T-cell immunotherapy for progressive MS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

