MRI offers very high sensitivity to tissue microstructural damage and generally has a high resolution; PET has the highest possible specificity for single cellular, myelin, or neuronal targets, but at the expense of resolution, which is generally very low.
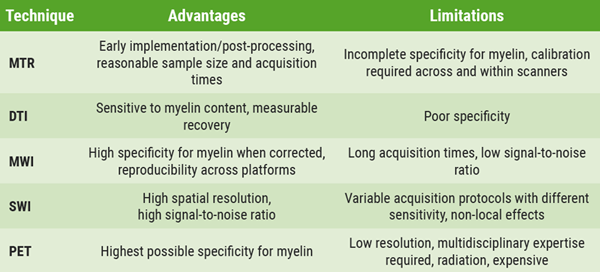
Dr Bodini gave an overview of MRI techniques that are sensitive to myelin content changes. Magnetisation transfer ratio (MTR) is very sensitive to myelin and has very reasonable acquisition times. However, the signal is affected by oedema and axonal density, as well as by microglia. MTR captures changes in myelin content in single lesions. Dr Bodini said MTR has already been shown to be sensitive to the effects of remyelinating treatment in clinical trials and that sample sizes are very reasonable. She added that inhomogeneous magnetisation transfer (ihMT) can improve MTR because of a higher myelin specificity. Other valuable techniques that can measure myelin changes include diffusion-weighted imaging (DWI), myelin water fraction imaging (MWI), and quantitative susceptibility-weighed imaging (SWI) to measure myelin and iron. As MRI techniques have a suboptimal specificity for myelin in vivo, PET holds a special place in imaging de-/remyelination. PET captures clinically relevant remyelination, which is critical to determine disease evolution and disability (see Table 1).
Table 1. Imaging techniques for de- and remyelination: a summary [1].
Dr Bodini went on to discuss imaging techniques to measure potential neuroprotection. The most widely used is whole brain atrophy, which is easy to implement and correlates with clinical and cognitive scores. Thalamic atrophy may be a promising primary MRI endpoint for phase 2 trials. Early markers of neuronal damage are N-acetyl-aspartate 1H-MRS (NAA-1H-MRS), advanced DWI, 11C-FMZ, and synaptic vesicle protein.
“A turning point in the search for effective imaging measures of neuroprotection will be the development of imaging strategies to evaluate the key mechanisms leading to neurodegeneration”, Dr Bodini argued. One of these mechanisms is energy dysregulation. Different techniques have been developed to investigate different aspects of energy dysregulation, notably 23Na-MRI, 31P-MRS, and diffusion-weighted (DW)-MRS (see Table 2).
Table 2. Imaging techniques for neuroprotection: a summary [1].

Dr Bodini concluded with the following take-home messages:
- MRI and PET should be deployed as outcome measures in future clinical phase 2 trials testing pro-myelinating and neuroprotective MS treatments.
- Future outcome measures will include imaging techniques of mechanisms leading to neurodegeneration.
- PET should be used in the future to validate single MRI sequences or a combination of multiple MRI measurements to improve MRI specificity for myelin and neurons.
- Bodini B. Symposium SYMP04, EAN 2020.
Posted on
Previous Article
« MRI-based clustering of MS patients Next Article
Long-term safety of satralizumab consistent with double-blind periods »
« MRI-based clustering of MS patients Next Article
Long-term safety of satralizumab consistent with double-blind periods »
Table of Contents: EAN 2020
Featured articles
Alzheimer's Disease and Other Dementias
Non-Alzheimer’s disease pathophysiology in the elderly
Novel genetic association with resistance to ERC tau deposition
Diastolic dysfunction novel risk factor for cognitive impairment
Epilepsy
Avoidable epilepsy-related mortality remains high
How genetic testing can contribute to epilepsy management
Cenobamate effective in focal epilepsy
Sustained seizure reductions with cannabidiol for Lennox-Gastaut syndrome
Prevalence of autoantibodies in epilepsy almost 10%
Parkinson's Disease
White matter matters in Parkinson’s disease
Sleep disorders mark PD progression
Directional DBS superior to omnidirectional DBS
Stroke
Benefits of statins to prevent stroke outweigh risks
Extubation after thrombectomy: the sooner, the better
Thrombus location and length predictors of early neurological deterioration
Endovascular treatment in large vessel occlusion stroke patients treated with OAC
Early edoxaban may be safe after cardioembolic stroke
Headache and Pain
Small fibre pathology as biomarker for fibromyalgia
Migraine as a cyclical functional disorder
Reassuring real-world safety profile of 3 CGRP inhibitors
Long-term cardiovascular safety of erenumab
Real-world data for erenumab in Germany
Eptinezumab in chronic migraine and medication-overuse headache
Fremanezumab tolerability in cardiovascular patients with migraine
Effects of galcanezumab on health-related quality of life
Multiple Sclerosis
Imaging to evaluate remyelination and neuroprotection
Serum NfL predicts long-term clinical outcomes in MS
Epstein-Barr virus-targeted T-cell immunotherapy for progressive MS
High NEDA rates after 2 years of ocrelizumab
Switching from natalizumab to moderate- versus high-efficacy DMT
Results of compounds in late stages of development
Alemtuzumab efficacy and safety data of over 9 years
Fampridine treatment results in routine clinical practice
Air pollution is a possible risk factor for MS
Neuromyelitis Optica Spectrum Disorder
Genetic association studies in NMOSD needed
Eculizumab in NMOSD: the PREVENT study
Long-term safety of satralizumab consistent with double-blind periods
Neuromuscular Disorders
Biomarkers predicting motor function in SMA
Sustained benefits of avalglucosidase alfa in late-onset Pompe disease
Efficacy and safety of rituximab in refractory MG corroborated
Related Articles
September 9, 2020
Benefits of statins to prevent stroke outweigh risks
September 9, 2020
Cenobamate effective in focal epilepsy
September 9, 2020
Directional DBS superior to omnidirectional DBS
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

