EBV is considered a risk factor in triggering MS. Mounting evidence suggests that EBV-infected immune cells, in particular memory B cells, play an important role in propagating both relapsing and progressive forms of MS. ATA188 offers a novel treatment approach selectively targeting and eliminating EBV-infected B cells and plasma cells in the circulation and central nervous system.
A phase 1a multicentre study assessed safety and efficacy of ATA188 in patients with progressive forms of MS. Patients were treated across 4 dose-escalating cohorts, with 6 patients each in cohorts 1-3 and 7 patients in cohort 4. Across the 4 dose cohorts, ATA188 was well tolerated, with no dose-limiting toxicities or fatal adverse events. Additionally, ATA188 infusion showed no clinically meaningful effect on cytokine levels post-infusion.
Two methods to assess clinical outcomes were used. The first scale was based on Sustained Disability Improvement (SDI), a composite of improvement in Expanded Disability Status Scale (EDSS) or Timed 25-Foot Walk at consecutive time points (3 and 6 months, 6 and 12 months). All patients in cohorts 1-3 showing SDI at 6 months maintained improvement through 12 months (see Table). Additionally, there was a dose-related increase in the number of patients with SDI. The second composite scale (designed to detect early signals of efficacy) was an a priori classification of patient outcomes, incorporating 7 scales for MS symptoms, function, and disability. This scale also showed a dose-related trend of a higher proportion of patients showing favourable clinical improvement. Based on these results, the cohort 3 dose was selected for the randomised, placebo-controlled phase 1b study.
Table. Clinical outcomes and composite scale of SDI in patients receiving ATA188 [1].
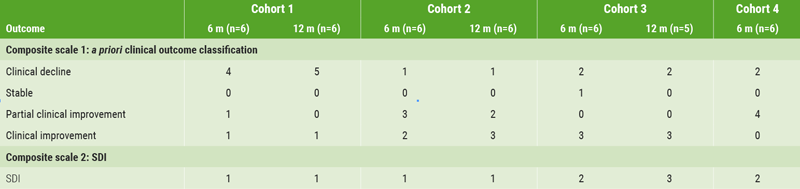
m, months; SDI, sustained disability improvement.
- Bar-Or A, et al. Abstract LB130, EAN 2020.
Posted on
Previous Article
« High NEDA rates after 2 years of ocrelizumab Next Article
Serum NfL predicts long-term clinical outcomes in MS »
« High NEDA rates after 2 years of ocrelizumab Next Article
Serum NfL predicts long-term clinical outcomes in MS »
Table of Contents: EAN 2020
Featured articles
Alzheimer's Disease and Other Dementias
Non-Alzheimer’s disease pathophysiology in the elderly
Novel genetic association with resistance to ERC tau deposition
Diastolic dysfunction novel risk factor for cognitive impairment
Epilepsy
Avoidable epilepsy-related mortality remains high
How genetic testing can contribute to epilepsy management
Cenobamate effective in focal epilepsy
Sustained seizure reductions with cannabidiol for Lennox-Gastaut syndrome
Prevalence of autoantibodies in epilepsy almost 10%
Parkinson's Disease
White matter matters in Parkinson’s disease
Sleep disorders mark PD progression
Directional DBS superior to omnidirectional DBS
Stroke
Benefits of statins to prevent stroke outweigh risks
Extubation after thrombectomy: the sooner, the better
Thrombus location and length predictors of early neurological deterioration
Endovascular treatment in large vessel occlusion stroke patients treated with OAC
Early edoxaban may be safe after cardioembolic stroke
Headache and Pain
Small fibre pathology as biomarker for fibromyalgia
Migraine as a cyclical functional disorder
Reassuring real-world safety profile of 3 CGRP inhibitors
Long-term cardiovascular safety of erenumab
Real-world data for erenumab in Germany
Eptinezumab in chronic migraine and medication-overuse headache
Fremanezumab tolerability in cardiovascular patients with migraine
Effects of galcanezumab on health-related quality of life
Multiple Sclerosis
Imaging to evaluate remyelination and neuroprotection
Serum NfL predicts long-term clinical outcomes in MS
Epstein-Barr virus-targeted T-cell immunotherapy for progressive MS
High NEDA rates after 2 years of ocrelizumab
Switching from natalizumab to moderate- versus high-efficacy DMT
Results of compounds in late stages of development
Alemtuzumab efficacy and safety data of over 9 years
Fampridine treatment results in routine clinical practice
Air pollution is a possible risk factor for MS
Neuromyelitis Optica Spectrum Disorder
Genetic association studies in NMOSD needed
Eculizumab in NMOSD: the PREVENT study
Long-term safety of satralizumab consistent with double-blind periods
Neuromuscular Disorders
Biomarkers predicting motor function in SMA
Sustained benefits of avalglucosidase alfa in late-onset Pompe disease
Efficacy and safety of rituximab in refractory MG corroborated
Related Articles
September 10, 2020
Switching from natalizumab to moderate- versus high-efficacy DMT
September 10, 2020
Biomarkers predicting motor function in SMA
September 9, 2020
Prevalence of autoantibodies in epilepsy almost 10%
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

