https://doi.org/10.55788/8754b186
The study was testing the efficacy and safety of etrasimod in patients with moderate to severe ulcerative colitis, a chronic inflammatory disease of the colon that leads to ulcers causing abdominal pain, bloody stools and incontinence.
Inflammatory bowel disease is a $20 billion market globally, making it a lucrative target for drugmakers.
Etrasimod met the main goal of statistically significant improvement in remission at week 12 compared to placebo. Statistically significant improvements were achieved in all key secondary goals in the trial as well, the company said in a press release (https://bit.ly/3tzZGNi).
By Reuters Staff
Posted on
Previous Article
« Metastasis rate high after endoscopic resection of high-risk T1a esophageal cancers Next Article
Parkinson’s gene signature in memory T cells a potential new therapeutic target »
« Metastasis rate high after endoscopic resection of high-risk T1a esophageal cancers Next Article
Parkinson’s gene signature in memory T cells a potential new therapeutic target »
Related Articles

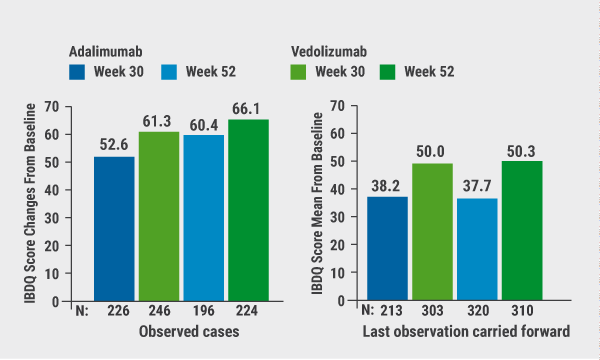
April 14, 2020
Effects of vedolizumab versus adalimumab on QoL
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

