https://doi.org/10.55788/22b41947
As Prof. Diamant Thaci (University of Lübeck, Germany) pointed out, previously, researchers focused more on pathogenesis, whereas in the future, the phenotype of the patients is expected to play an important role [1].
It is crucial to recognise generalised pustular psoriasis (GPP), a rare type of psoriasis. Frequently it presents with flares of widespread sterile pustules on a background of red and tender skin. IL-36 plays a key role in the pathogenesis of the disease. Published phase 2 data of spesolimab, an anti-IL-36 receptor antibody, showed that more than half of the participants achieved complete clearance of pustules with a single dose of the drug within a week [2]. Imsidolimab is another anti-IL-36 receptor monoclonal antibody that led to rapid and sustained improvement in GPP signs and symptoms, in a phase 2 trial presented at the previous European Academy of Dermatology and Venereology (EADV) congress [3]. In Prof. Thaci's view, the efficacy of the anti-IL-36 agents will be not an issue but safety. “This will be crucial for establishing these agents as a treatment option,” he said.
A novel treatment option for plaque psoriasis is the dual blockade of IL-17A and IL-17F by bimekizumab, which is supposed to be more efficient than the blockade of IL-17A only. Indeed, in the phase 3b trial BE RADIANT, treatment with bimekizumab resulted in greater skin clearance (PASI100 response) than treatment with secukinumab over 16 and 48 weeks [4]. “We will see in registries whether similar data is found in the daily practice,” Prof. Thaci said. An unexpected result of the trial program with bimekizumab was that PASI100 achieved at 16 weeks could be maintained over 2 years. “Finally, this agent also has a scratch: patients treated with bimekizumab get more candidiasis. How important this is in daily practice will be shown in the future,” Prof. Thaci explained.
Another novel treatment option is sonelokimab, an anti-IL-17A/F nanobody. This agent blocks the homodimers of IL-17A and IL-17F and the heterodimer IL-17A/F. In a phase 2 dose-finding trial, this agent showed an excellent treatment response, with rates for PASI90 and PASI100 ranging from 79.2% to 90.4% and 40.4% to 56.9%, respectively [5].
Effective oral therapy on the horizon
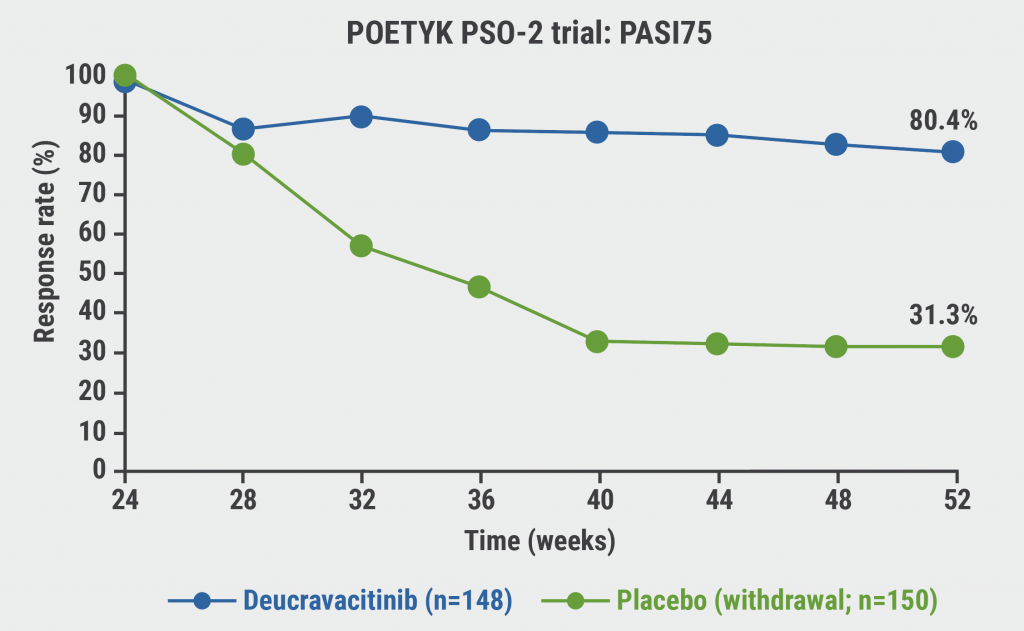
“In atopic dermatitis, we have a JAKmania, but in psoriasis, we have a TYKmania,” Prof. Thaci said. Deucravacitinib is an oral TYK2 inhibitor that selectively inhibits TYK2 via an allosteric mechanism by uniquely binding to the regulatory domain rather than to the active domain where JAK1/2/3 inhibitors bind. This unique binding provides high functional selectivity for TYK2, which might have advantages regarding tolerability [6]. “Deucravacitinib is more effective than apremilast, which is not a high hurdle. But what is interesting is that if you stop treatment, patients maintain PASI75 for 6 months (see Figure). This has never happened before with an oral drug: this is a new quality. There is a place for oral therapy in psoriasis,” Prof. Thaci said [7]. In addition, there will be a couple of novel topical preparations. “If you were expecting nothing on the horizon for psoriasis, I must tell you that there is,” Prof. Thaci concluded.
Figure: Maintenance of PASI75 response through week 52 following deucravacitinib withdrawal [7]
- Thaci, D. Systemic treatment for psoriasis – What is on the horizon? FS4, SPIN 2022 Congress, 06–08 July, Paris, France.
- Bachelez H, et al. New Engl J Med. 2021;385:2431-40.
- Gudjonsson J, et al.06, EADV 2021 Virtual Congress, 29 Sept–2 Oct.
- Reich K, et al. New Engl J Med. 2021;385:142-52.
- Papp KA, et al. Lancet. 2021;397:1564-1575.
- Chimalakonda A, et al. Dermatol Ther. 2021;11:1763–1776.
- Warren RB. D1T01.4C, EADV 2021 Virtual Congress, 29 Sept–2 Oct.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Topical therapy in psoriasis: an important partner in combination therapy Next Article
No adverse pregnancy outcomes in patients exposed to baricitinib »
« Topical therapy in psoriasis: an important partner in combination therapy Next Article
No adverse pregnancy outcomes in patients exposed to baricitinib »
Table of Contents: SPIN 2022
Featured articles
Letter from the Editor
IMIDs in Adults and Children: New Developments
Therapies for atopic dermatitis: still moving forward
Children with AD: high risk of bacterial infections in carriers of a filaggrin gene variant
Men on biologics report fewer adverse events than women
Conceptual framework of adverse drug reactions may improve treatment of patients with IMIDs
Psoriasis: The Beat Goes On
Systemic treatment for psoriasis: what is on the horizon?
Topical therapy in psoriasis: an important partner in combination therapy
GPP flares: pronounced undertreatment is common
IL-17A/F inhibitor bimekizumab shows higher response and maintenance rates compared with secukinumab
Paediatric psoriasis: ixekizumab beneficial in difficult-to-treat areas
Psoriasis patients see great benefit in achieving complete skin clearance
The Future Is Bright for Vitiligo
Predilection sites for skin signs of vitiligo disease activity determined
Where Are We Now in Hidradenitis Suppurativa
IHS4 better suited as an outcome measure in HS trials?
New treatments for HS: IL-17 inhibitors next in practice?
New Treatment Options in Alopecia Areata
Alopecia areata: light at the end of the tunnel
Alopecia areata pathogenesis: known genetic background, unknown environmental triggers
Best of the Posters
Psoriasis treatment: no elevation of MACE and VTE on deucravacitinib
Comorbid anxiety and depression may benefit from psoriasis treatment with certolizumab
Dose tapering in psoriasis is associated with a low relapse rate
Related Articles
February 4, 2020
Lowest risk of infection after therapy with an IL-12/IL-23 blocker
February 2, 2022
TNF blockers likely beneficial for psoriatic patients with COVID-19
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

