https://doi.org/10.55788/70574b24
Unlike in psoriasis and atopic dermatitis, the inflammatory element of hidradenitis suppurativa (HS) advances [1]. “We have a disease that progresses from an inflammatory component to a scarring component if the inflammatory part is not treated well and early enough,” Prof. Thrasyvoulos Tzellos (Nordland Hospital Trust, Norway) stated. Up to now, adalimumab is the only approved biological treatment, thus, there is still a high unmet need. Still, there is somewhat unimpressive data on drug survival rates of adalimumab in HS (56.3% at 1 year and 30.5% at 2 years), with ineffectiveness being the main reason for discontinuation. [2]. Currently, the common treatment goal is a 50% reduction in the HiSCR score [1]. “Do we need higher threshold outcomes in order to depict higher inflammatory effects?” Prof. Tzellos asked. As phase 2 data from bimekizumab (NCT03248531) showed that the use of higher HiSCR thresholds was able to demonstrate drug efficiency and simultaneously reduce the rate of placebo response (see Figure), his answer was ‘yes’ [1,3].
Figure: Is there a potential to aim for more ambitious HiSCR thresholds? [3]
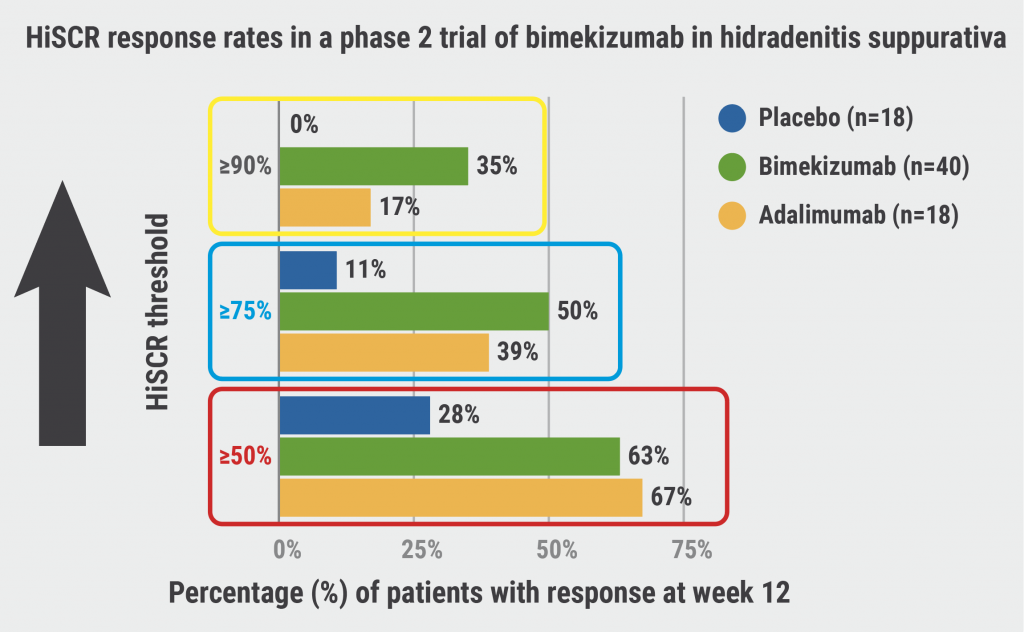
HiSCR, Hidradenitis Suppurativa Clinical Response.
Prof. Tzellos continued that using HiSCR involves an inherent problem, as it only measures a reduction in the total count of abscesses and nodules but disregards draining tunnels. This may lead to study results in which participants who reach HiSCR100 still have actively draining tunnels. A validated score that captures all the components is the IHS4 score [4]. It includes draining tunnels and assigns different weights to the different types of lesions: nodules are multiplied by 1, abscesses by 2, and draining tunnels by 4. A post-hoc analysis of individual patient data from the PIONEER 1 and 2 trials (NCT02906930 and NCT01468233) that used the IHS4 score led to decreased placebo response rates between 4.5% and 12% [1,5]. “Actually, it reduced it to the rates we are familiar with in psoriasis and atopic dermatitis,” Prof. Tzellos commented. Further analysis of PIONEER 1 and 2 data by the Hidradenitis Suppurativa Foundation identified and externally validated IHS4-55 as an important dichotomous outcome [1]. “IHS4-55 will replace HiSCR because it has the same abilities when it comes to discriminating treatment groups, but it does not have the same drawbacks HiSCR has, as it includes dynamically draining tunnels in a validated manner,” Prof. Tzellos summarised.
- Tzellos T. Hidradenitis Suppurativa: Pipeline and new concepts. FS4, SPIN 2022 Congress, 06–08 July, Paris, France.
- Prens LM, et al. Br J Dermatol. 2021;185:177-84.
- Glatt S, et al. JAMA Dermatol. 2021;157:1279-88.
- Zouboulis CC, et al. Br J Dermatol. 2017;177:1401-09.
- Frew JW, et al. J Am Acad Dermatol. 2020;82:1150-57.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« New treatments for HS: IL-17 inhibitors next in practice? Next Article
Vitiligo-childhood onset commonly under 10 years »
« New treatments for HS: IL-17 inhibitors next in practice? Next Article
Vitiligo-childhood onset commonly under 10 years »
Table of Contents: SPIN 2022
Featured articles
Letter from the Editor
IMIDs in Adults and Children: New Developments
Therapies for atopic dermatitis: still moving forward
Children with AD: high risk of bacterial infections in carriers of a filaggrin gene variant
Men on biologics report fewer adverse events than women
Conceptual framework of adverse drug reactions may improve treatment of patients with IMIDs
Psoriasis: The Beat Goes On
Systemic treatment for psoriasis: what is on the horizon?
Topical therapy in psoriasis: an important partner in combination therapy
GPP flares: pronounced undertreatment is common
IL-17A/F inhibitor bimekizumab shows higher response and maintenance rates compared with secukinumab
Paediatric psoriasis: ixekizumab beneficial in difficult-to-treat areas
Psoriasis patients see great benefit in achieving complete skin clearance
The Future Is Bright for Vitiligo
Predilection sites for skin signs of vitiligo disease activity determined
Where Are We Now in Hidradenitis Suppurativa
IHS4 better suited as an outcome measure in HS trials?
New treatments for HS: IL-17 inhibitors next in practice?
New Treatment Options in Alopecia Areata
Alopecia areata: light at the end of the tunnel
Alopecia areata pathogenesis: known genetic background, unknown environmental triggers
Best of the Posters
Psoriasis treatment: no elevation of MACE and VTE on deucravacitinib
Comorbid anxiety and depression may benefit from psoriasis treatment with certolizumab
Dose tapering in psoriasis is associated with a low relapse rate
Related Articles
September 17, 2021
PsoBarrier EU: Large survey evaluating quality of care in Europe
February 4, 2020
Lowest risk of infection after therapy with an IL-12/IL-23 blocker
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

