The presented data compared molecular and histopathologic patterns in psoriatic skin from the phase 3 UltiMMa-1 trial (NCT02684370), a head-to-head comparison of treatment with risankizumab and ustekinumab for moderate-to-severe plaque psoriasis [1]. The 2 monoclonal antibodies bind to different subunits of IL-23: risankizumab to p19 and ustekinumab to p40, a subunit that is shared by IL-23 and IL-12 [2,3].
In UltiMMa-1, participants received either 150 mg of risankizumab or 45/90 mg of ustekinumab at weeks 0, 4, 16, 28, and 40 [1]. From consenting participants, skin biopsies were also obtained at baseline in lesional and non-lesional skin and at weeks 12 and 46 in lesional skin. “The analysis methods were histopathological analysis of sections stained with hematoxylin and eosin, and immunohistochemistry performed for the proliferation marker Ki67, the T-cell marker CD3, the dendritic cell markers CD11c and DC-LAMP, which stains matured dendritic cells,” Dr Kathleen Smith (AbbVie Bioresearch Center, MA, USA) explained. Gene expression in the biopsies was analysed using RNA sequencing.
The 2 study arms were fairly similar in terms of mean age (53 and 51 years), body mass index (31 and 30 kg/m2), and percentage of women (33% and 38%). However, the involved body surface area was 27.3% in the risankizumab and 18.8% in the ustekinumab group.
At weeks 12 and 46, both agents significantly decreased the number of cells positive for CD3, CD11c, DC-LAMP, and Ki67. At week 12, a difference in histopathology scores was found between risankizumab and ustekinumab treated patients. On risankizumab, 76% achieved an excellent or likely excellent improvement versus 45% on ustekinumab. “These improvements were maintained by week 46,” Dr Smith further indicated.
The transcriptome analysis included participants with a Psoriasis Area Severity Index (PASI) 90 response at week 46. “We defined the psoriasis transcriptome as the 3,146 genes which were differentially expressed in lesional versus non-lesional skin at baseline,” Dr Smith stated. Risankizumab therapy led to 79% changes in the transcriptome and ustekinumab in 69%, respectively. A more detailed look at transcriptome modulation at week 12 revealed that the 2 agents downregulated, for example, CCL20, FoxP3, IL-17A, IL-23A, and the IL-36 transcripts. “However, there is a large group of genes that were modified only by risankizumab and these included T-cell gene CD28, CD3, activation marker CD69, and CD 8 itself (see Figure). This supports the histological analysis where we see slightly better improvement in T-cell infiltration,” Dr Smith highlighted. Treatment with risankizumab also downregulated the kinetochore metaphase signalling pathway and the role of IL-17A in psoriasis (see Figure).
Figure: Modulation of the psoriasis transcriptome on treatment with risankizumab or ustekinumab [1]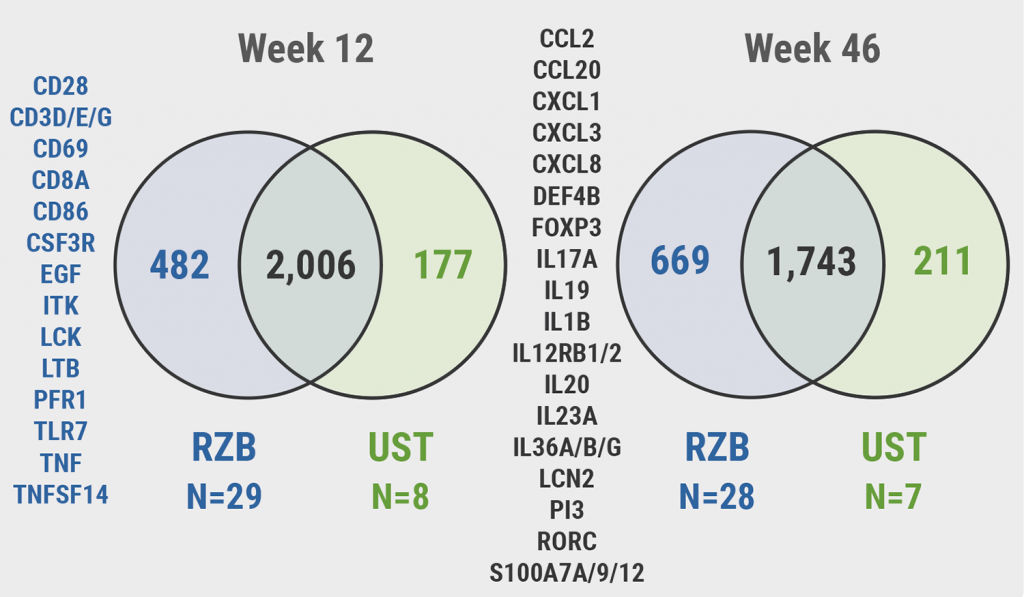 RZB, risankizumab; UST, ustekinumab.
RZB, risankizumab; UST, ustekinumab.
“A higher proportion of patients treated with risankizumab experienced an excellent improvement in histopathology scores compared with patients with ustekinumab, which parallels clinical efficacy, and treatment with risankizumab modulated a greater number of psoriasis transcriptome genes, including those specifically targeting inflammation pathways compared with ustekinumab,” Dr Smith concluded.
- Smith, K. Interleukin-23 pathway inhibition by risankizumab differentially modulates the molecular profile in psoriatic skin compared with ustekinumab. FC28, Psoriasis from Gene to Clinic 2021, 9–11 December.
- Wcisło-Dziadecka DL, et al. Adv Clin Exp Med. 2020;29(2):235–241.
- Strober B, et al. J Eur Acad Dermatol Venereol. 2020; 34(12):2830–2838.
Copyright ©2021 Medicom Medical Publishers
Posted on
Previous Article
« Tapering biologics: No alarming signs of increased anti-drug antibodies Next Article
Guselkumab shows highest drug survival among systemic treatments »
« Tapering biologics: No alarming signs of increased anti-drug antibodies Next Article
Guselkumab shows highest drug survival among systemic treatments »
Table of Contents: PFGC 2021
Featured articles
Letter from the Editor
Guselkumab shows highest drug survival among systemic treatments
Genes in Psoriasis and Psoriatic Arthritis
HLA-C*06:02-positive patients on ustekinumab show higher drug survival in a real-world scenario
Protective factors identified against anti-drug antibody formation to adalimumab in psoriasis
Comorbidity in Psoriasis
Psoriasis associated with a higher cancer risk
Comorbidity and clinical features of psoriasis vary according to HLA-C*06:02 status
Psoriasis patients with cardiovascular comorbidity characterised by high systemic inflammation
Psoriasis Therapy: New Findings
Inhibition of heat shock protein: A novel way to treat psoriasis?
Guselkumab shows highest drug survival among systemic treatments
Tapering biologics: No alarming signs of increased anti-drug antibodies
Intermediate monocytes are possible predictors of response to secukinumab
Gut microbiota of psoriasis patients: less diverse and reduced functionality
COVID-19: What's New
DLQI scores underestimated during lockdowns?
TNF blockers likely beneficial for psoriatic patients with COVID-19
Patients on immunomodulators need 2 COVID-19 vaccinations before seroconversion
Paradoxical Reactions to Biologics
The Yin and Yang of opposing vectors: an explanation for side effects of biologics
Explaining arthropathy development through IL-4 and IL-13 blockade
Best of the Posters
Potential biomarker discovered for treatment response to ustekinumab
TNF inhibitor for immune-mediated inflammatory disease doubles the risk of paradoxical psoriasis
Secukinumab also tolerable in paediatric psoriasis patients
High treatment success with ixekizumab in patients with psoriasis and diabetes
Related Articles
June 21, 2019
Atopic dermatitis and psoriasis: on a spectrum?
October 20, 2023
Analysing the Link Between Psoriasis and Chronic Kidney Disease
December 17, 2020
Promising results with nanobody treatment in psoriasis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

