https://doi.org/10.55788/6cec6db7
“AA has started a new area of treatment with the JAK inhibitors. However, a remaining unmet need is a drug with a benefit/risk profile allowing early intervention, especially in the paediatric population,” said Prof. Ulrike Blume-Peytavi (Charité – Berlin University Medicine, Germany) [1]. Early intervention after the first manifestation in infancy might prevent further disease progression or chronicity.
AA is driven by the trafficking of cytotoxic lymphocytes to the peri-follicular and intra-follicular regions, mediated by adhesion molecules. The botanical drug solution coacillium (22.25%) contains extracts of lemon, onion, cocoa, and guarana. The drug reduces pro-inflammatory adhesion molecules; thus, counteracting the immune privilege collapse typical for AA and has also an effect on the apoptotic pathway. This was the rationale for assessing the efficacy and safety of this solution in children and adolescents with moderate-to-severe AA.
Prof. Blume-Peytavi presented the randomised, double-blind, multicentre, phase 2/3 trial RAAINBOW (NCT03240627), which included AA patients aged 2–18 years with a Severity of Alopecia Tool (SALT) score of 25–50 (corresponding to moderate AA) and 50–95 (severe AA). They were randomly assigned to the botanical solution (22.5%) twice daily or placebo and treated for 24 weeks. This period was followed by a treatment-free period of 24 weeks to evaluate disease relapse after treatment discontinuation.
At 24 weeks, 62 participants with an average age of 11 years could be analysed (42 in the intervention arm and 20 in the placebo arm). Treatment with the botanical solution led to the primary endpoint of a mean change in SALT score of 22.9% in the coacillium group versus -8.0% in the placebo group (P<0.0001). Moreover, 26.2% in the coacillium group achieved a 40% relative reduction in SALT after 24 weeks of treatment compared with 5% in the placebo group.
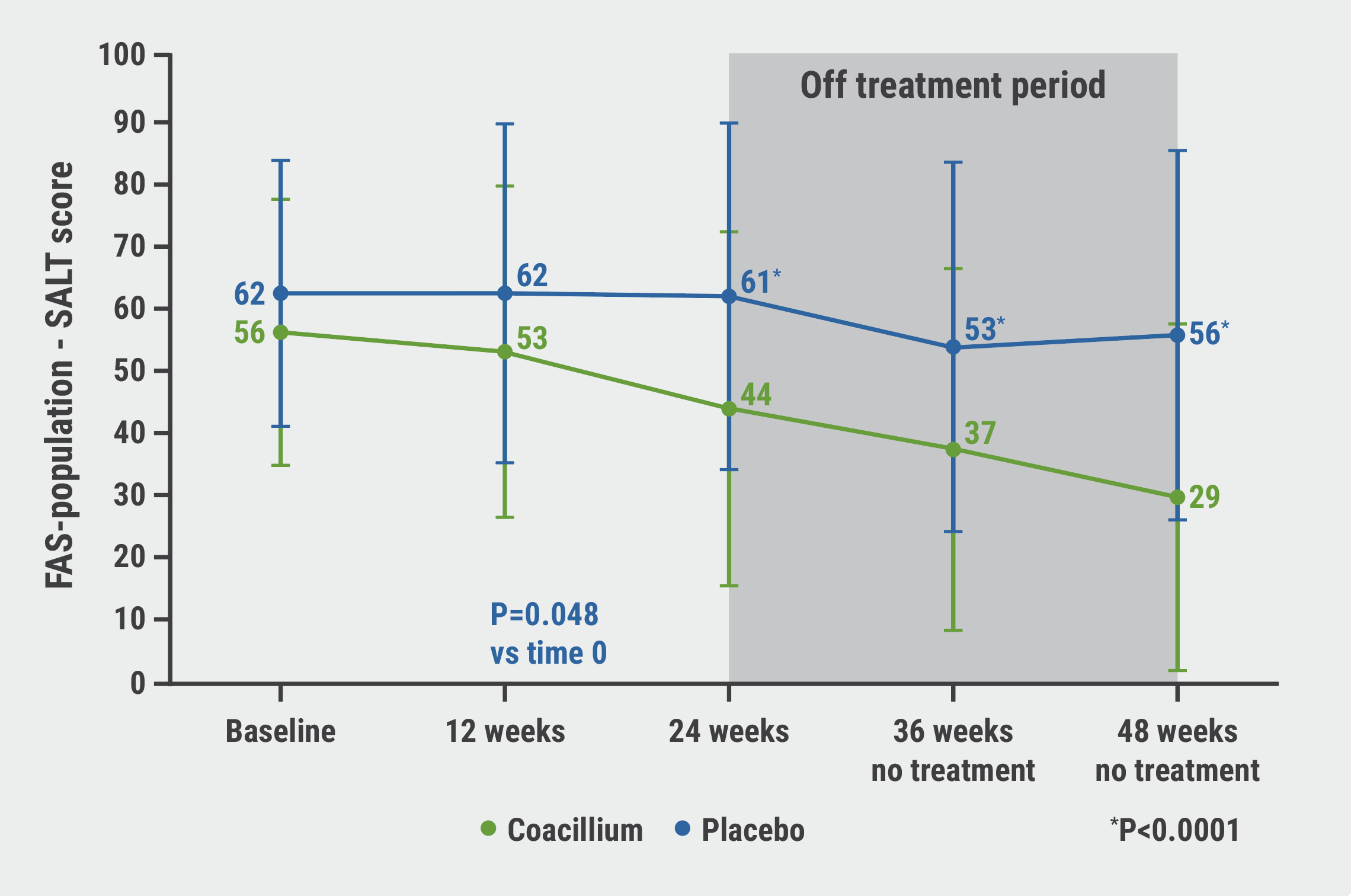
The SALT score of the botanical solution-treated participants continued to improve after treatment discontinuation, from 44 to 29 (see Figure). After discontinuation, 82% of participants treated with the botanical solution experienced hair growth after treatment discontinuation. At week 48, almost half of the participants (47%) in the intervention arm reached SALT scores ≤20 compared with 9.1% in the placebo group (P=0.0031).
Figure: After coacillium discontinuation at week 24, SALT score continues to improve from 44 to 29 [1]
The solution was well tolerated with only local transient, mild, or moderate side effects, except for 1 case of severe transient eczema that stopped after treatment discontinuation.
- Blume-Peytavi U, et al. Efficacy and safety of coacillium in children and adolescents with moderate to severe alopecia areata: a randomized, double-blind, multicentre, phase 2-3 trial. D1T01.1L, EADV Congress 2023, 11–14 October, Berlin, Germany.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Nemolizumab shows high success rates in prurigo nodularis Next Article
Alopecia areata: remarkable regrowth rates with deuruxolitinib »
« Nemolizumab shows high success rates in prurigo nodularis Next Article
Alopecia areata: remarkable regrowth rates with deuruxolitinib »
Table of Contents: EADV 2023
Featured articles
Tapinarof on course to become a new topical treatment in AD
AD and Eczema in 2023
Tapinarof on course to become a new topical treatment in AD
Upadacitinib provides sustained skin clearance in adolescents and adults with AD
Sustained deep clinical and itch responses with novel IL-13 inhibitor
IL-13 inhibitor shows potential in atopic dermatitis
Encouraging results for amlitelimab in atopic dermatitis
Chronic hand eczema: patients share similar molecular signatures regardless of AD status
Severe hand eczema: dupilumab could be a future treatment
Psoriasis News
Dual IL-17 blockade yields efficacy on joints and skin
High-dose subcutaneous spesolimab prevents GPP flares up to week 48
Drug survival of guselkumab and risankizumab seems superior to other biologics
IL-23 blockers may lower the risk of developing inflammatory and psoriatic arthritis
First-in-class oral IL-23 inhibitor safe and effective for moderate-to-severe plaque psoriasis
Hidradenitis Suppurativa: End of the Diagnostic and Therapeutic Draught
Skin tape stripping allows a novel precision medicine approach in HS
Nanobodies: A novel way to treat HS
Anti-IL17 blockade leads to maintained pain reduction in patients with HS
Vitiligo: Novel Treatment Options
JAK1 inhibition: a promising forthcoming treatment option in vitiligo
Vitiligo: Continuation of topical ruxolitinib successful in many initial non-responders
Alopecia Areata: Novel Developments
JAK3/TEC inhibition achieves clinically meaningful responses in AA
Alopecia areata: remarkable regrowth rates with deuruxolitinib
Botanical drug solution improves hair regrowth in children and adolescents with AA
What’s New in Other Disease Entities
Nemolizumab shows high success rates in prurigo nodularis
Remibrutinib reduces itch, sleep problems, and activity impairment in patients with CSU
Innovative wound gel reduces frequency of painful dressing changes in epidermolysis bullosa
Best of the Posters
Women with psoriasis face increased adverse effects with systemic therapy
Improved AI tool shows high sensitivity rates in skin cancer detection
Dermoscopy training combined with AI significantly improves skin cancer detection
Related Articles
November 16, 2022
Baseline ctDNA predicts survival in resected stage III–IV melanoma
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

