https://doi.org/10.55788/4b955d09
A 2014 study taught the community much about the pathogenesis of AA, showing that the disease is driven by cytotoxic T lymphocytes and reversed by JAK inhibition [2]. “Since then, various studies have been conducted to assess the safety and efficacy of JAK inhibitors in the treatment of AA, including 5 randomised clinical trials.”
In a phase 2 study, ruxolitinib 1.5% cream failed in a population of patients with severe AA [3]. Similarly, a delgocitinib ointment was ineffective in a population of patients with AA [4]. Treatment with deuruxolitinib did result in a SALT score ≤20 in 30–42% of the patients after 24 weeks of therapy, meeting the primary endpoint of a phase 3 trial (NCT04518995) [5]. Likewise, long-term data of the phase 3 ALLEGRO-LT study investigating ritlecitinib showed that 30–40% of the patients achieved a SALT score ≤20 after 48 weeks of therapy (NCT04006457) [6]. Dr King added that the efficacy rates continued to improve after 24 weeks, indicating that it takes time to regrow hair in patients with AA. Finally, treatment with baricitinib resulted in an efficacy rate of 20–34% after 36 weeks of therapy, which rose further to 23–39% after 52 weeks of treatment in the BRAVE-AA1 and BRAVE-AA2 trials [7,8]. “In all these clinical trials, the vast majority of patients who achieved a SALT score ≤20 also achieved a SALT score ≤ 10,” mentioned Dr King.
Importantly, the trials showed that JAK inhibitors are less effective in patients with baseline SALT scores between 95 to 100 (10–20% efficacy) than in patients with baseline SALT scores between 50 and 94 (33–48% efficacy) [9]. Next, the duration of the current episode of severe disease influences the efficacy of JAK inhibition, with longer periods of hair loss (>4 years) resulting in reduced efficacy of JAK inhibitors compared with a shorter duration of the current episode of hair loss (≤4 years) [1]. “Thus, treating early appears to be very important,” added Dr King.
New treatment algorithm and oral minoxidil for AA
Dr King also drew attention to a forgotten agent. “Although the data is not as strong as the emerging data for JAK inhibitors, oral minoxidil demonstrated in 1987 to regrow hair in approximately 20% of patients with AA; yet, we have not been using this agent,” he said. Combining minoxidil with a JAK inhibitor may result in greater efficacy than either one of the agents as monotherapy, retrospective data suggests [10].
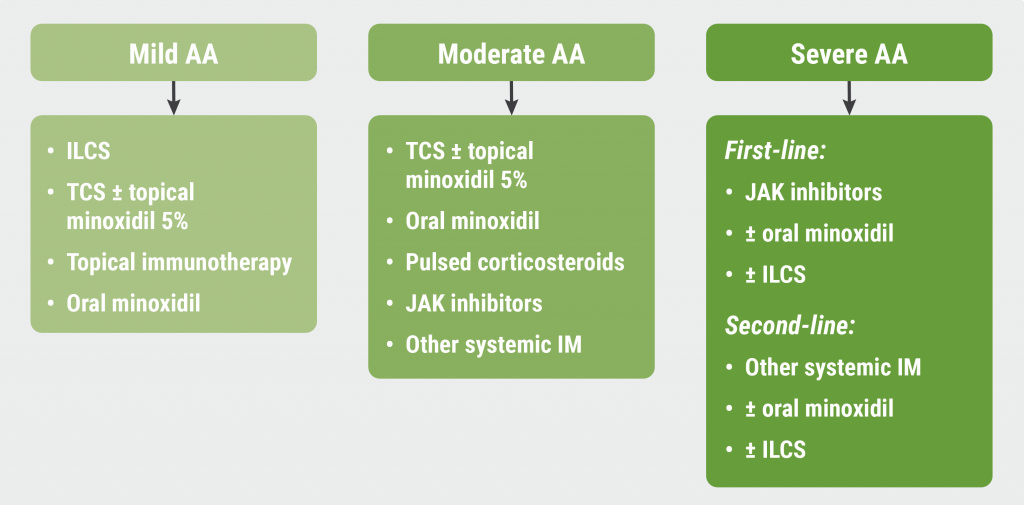
Finally, Dr King introduced a treatment algorithm for AA, discriminating between mild, moderate, and severe AA (see Figure). “JAK inhibitors won’t be changing the treatment for patients with mild AA, but JAK inhibitors are inarguably the first-line therapy for patients with severe disease,” he concluded.
Figure: Proposed treatment algorithm for alopecia areata [1]
- King B. JAK-inhibitors for alopecia areata and vitiligo. Blok 3, Dermatologendagen 2023, 9–10 March, Ermelo, the Netherlands.
- Xing L, et al. Nat Med. 2014;20:1043–1049.
- Olsen EA, et al. J Am Acad Dermatol. 2020;82(2):412–419.
- Mikhaylov D, et al. Arch Dermatol Res. 2023;315(2):181–189.
- King B. D3T01.1L, EADV Congress 2022, 7–10 September, Milan, Italy.
- Tsianakas A. D3T01.1G, EADV Congress 2022, 7‒10 September, Milan, Italy.
- King B, et al. N Engl J Med 2022;386:1687–1699.
- Kwon O, et al. Am J Clin Dermatol. 2023 Mar 1;1–9. Doi: 10.1007/s40257-023-00764-w.
- Taylor SC, et al. JAAD. 2022;87(3): Supplement AB52.
- Wambier CG, et al. JAAD. 2021;85(3):743–745.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Obesity and the skin: state of affairs Next Article
Eligibility and selection of JAK inhibitors for constitutional eczema »
« Obesity and the skin: state of affairs Next Article
Eligibility and selection of JAK inhibitors for constitutional eczema »
Table of Contents: DDD 2023
Featured articles
Dermato-Oncology
The role of surgeons in stage I–II melanoma
Melanoma: Surveillance and follow-up
When to screen for anal intraepithelial neoplasia?
JAK Inhibitors
Eligibility and selection of JAK inhibitors for constitutional eczema
Nutrition and the Skin
Obesity and the skin: state of affairs
What's New?
The importance of anti-microbial proteins in the skin
OCT non-inferior to biopsy in basal cell carcinoma
Infections
Scabies: Therapy failure and tips for the clinic
Related Articles
May 10, 2023
Scabies: Therapy failure and tips for the clinic
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

