https://doi.org/10.55788/5a23053c
The results of the phase 2 HALO study (NCT05137002) were highly anticipated since there have not been any new agents to treat uncontrolled hypertension in well over a decade, said Dr Deepak Bhatt (Icahn School of Medicine at Mount Sinai, NY, USA) [1]. Baxdrostat is a highly selective aldosterone synthase inhibitor. Its efficacy and safety were evaluated in the randomised, double-blind, placebo-controlled, multicentre, phase 2 HALO trial. The participants had to be on a stable regimen of an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB), an ACEi/ARB plus a thiazide diuretic, or an ACEi/ARB plus a calcium channel blocker, and have a mean seated SBP ≥140 mmHg. The primary endpoint was change from baseline in mean seated SBP after 8 weeks of treatment. A total of 249 patients were randomised 1:1:1:1 to baxdrostat 0.5 mg, 1 mg, 2 mg, or placebo; 227 completed the trial.
The primary endpoint was not met with any baxdrostat dose. SBP change was -17.0, -16.0, and -19.8 mmHg in the 0.5 mg, 1 mg, and 2 mg groups, respectively, but there was also a large decrease (-16.6 mmHg) in the placebo group (see Figure).
Figure: Placebo-corrected change from baseline in mean seated SBP at 8 weeks [1]
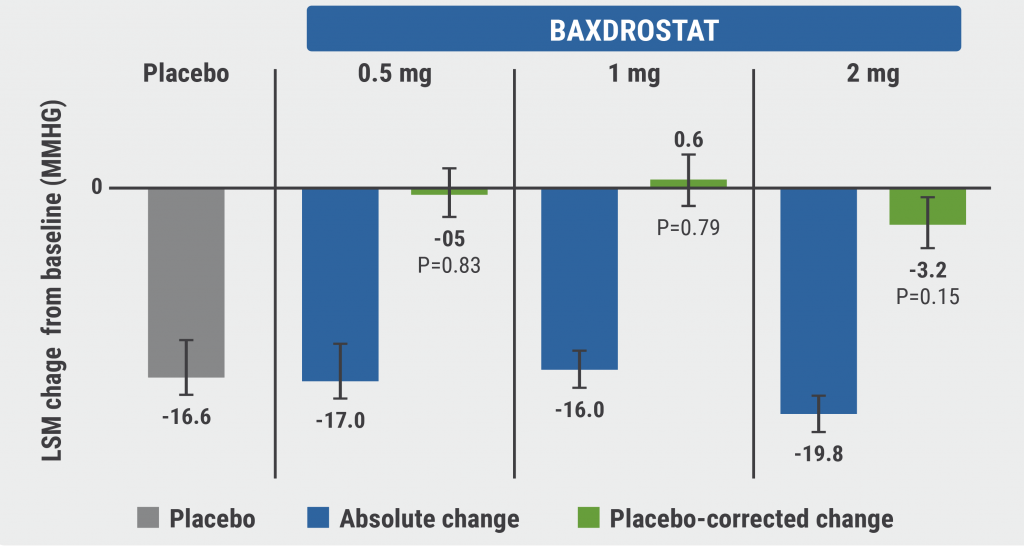
Similarly, none of the baxdrostat doses were associated with a significant difference in placebo-corrected diastolic blood pressure (DBP), which was a secondary endpoint. Change in DBP at 8 weeks did not significantly differ either; nor did the percentage of patients achieving a seated SBP response <130 mmHg at 8 weeks. Baxdrostat significantly reduced serum aldosterone levels at all doses. The treatment was well tolerated.
Dr Bhatt explained that a post-hoc analysis was done in patients who were adherent to the highest baxdrostat dose of 2 mg. The reason was that 20 of 54 (36%) patients in the 2 mg arm who completed 8 weeks of treatment had baxdrostat levels <0.2 ng/dL, indicating non-adherence (despite dosing records by pill count showing >95% adherence in all groups). This subgroup analysis suggested a placebo-corrected SBP reduction of 7.9 mmHg in patients adherent to baxdrostat 2 mg.
- Bhatt DL, et al. Results from a phase 2, double-blind, placebo-controlled trial evaluating the efficacy and safety of baxdrostat in patients with uncontrolled hypertension. Session 403-08, ACC Scientific Session 2023, 4–6 March, New Orleans, USA.
Copyright ©2023 Medicom Medical Publishers
Posted on
Previous Article
« Hormone therapy for gender dysphoria associated with increased CV risk Next Article
Oral PCSK9 inhibitor significantly lowers LDL-C »
« Hormone therapy for gender dysphoria associated with increased CV risk Next Article
Oral PCSK9 inhibitor significantly lowers LDL-C »
Table of Contents: ACC 2023
Featured articles
Pulmonary Arterial Hypertension
Sotatercept improves exercise capacity in patients with PAH
Fixed-dose macitentan plus tadalafil superior to either agent alone in PAH
Coronary Revascularisation
Immediate complete revascularisation non-inferior to staged complete revascularisation
RENOVATE-COMPLEX-PCI results support intravascular-guided PCI for complex lesions
Heart Failure and Cardiomyopathy
No need to restrict vigorous exercise in selected HCM patients?
No difference in CV outcomes between PET or CMR and SPECT
Interventional and Structural Cardiology
Benefits of MitraClip sustained to 5 years in COAPT trial
Transcatheter repair for patients with tricuspid regurgitation
Minimally invasive versus conventional sternotomy for mitral valve repair
Durable benefits of TAVR versus surgical aortic valve replacement in aortic stenosis patients
PCI not better than GDMT in severe ischaemic cardiomyopathy
Prevention
Anticoagulation in non-critically ill hospitalised COVID patients
Statins associated with reduced heart dysfunction from anthracyclines
Multifaceted strategy improves prescription of therapies for diabetes and ASCVD
Dyslipidaemia
Bempedoic acid benefits statin-intolerant patients at high cardiovascular risk
Evolocumab improves coronary plaque morphology in stable CAD
Inflammation stronger predictor of MACE than cholesterol levels
Oral PCSK9 inhibitor significantly lowers LDL-C
Miscellaneous
Baxdrostat in patients with uncontrolled hypertension
Hormone therapy for gender dysphoria associated with increased CV risk
Pulsed-field ablation appears safe and effective for atrial fibrillation
Key correlates of incident dementia identified in the MESA study
Related Articles
June 16, 2021
Monoclonal antibody rapidly reduces brain amyloid
February 4, 2022
Alzheimer’s-like changes seen in COVID-19 patients’ brains
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

