Oliver
Ottmann
× Oliver
Ottmann
* First author
Affiliation
Emeritus Professor, Cardiff University, UK
* First author
Affiliation
Emeritus Professor, Cardiff University, UK
Doi
https://doi.org/10.55788/5f751f03
INTRODUCTION
The past few years have seen advances in the therapeutic management of B-lineage acute lymphoblastic leukaemia (ALL) that border on the revolutionary, with paradigm shifts in relation to the use of chemotherapy, treatment intensity, hemopoietic cell transplantation (HCT) and utilization of targeted therapies. Concerning the latter, it is convenient to distinguish time periods before and after the introduction of immunotherapies, specifically monoclonal antibodies targeting cell surface antigens (inotuzumab ozogamicin), bispecific antibodies (blinatumomab) and more recently CAR T-cell therapy. For Philadelphia chromosome-positive (Ph+)/BCR::ABL1-positive ALL, we can additionally delineate a pre- and post-tyrosine kinase inhibitor (TKI) era.
Lessons and questions from the TKI era
ABL1-directed TKI were the first class of targeted agents that proved to be game changers; successive generations of TKI were able to induce – as single agents or in combination with corticosteroids - a complete remission in nearly all patients diagnosed with Ph+ ALL. This, plus a substantially lower toxicity and early death rate with improved outcomes facilitated reduced-intensity induction treatment as a new therapeutic principle.1-3 Moreover, the use of TKI in combination with established post-remission regimens (chemotherapy and HCT) resulted in survival outcomes equivalent to those of patients with Philadelphia-negative ALL. 4
As a caveat, the concept of using TKI alone in combination with substantially reduced intensity chemotherapy applies to induction therapy for Ph+ ALL, but not to consolidation cycles, at least in the absence of immunotherapy. This was demonstrated by an increased relapse rate in patients in whom high-dose cytarabine was omitted from consolidation therapy in a recent randomised, nilotinib-based trial (Graaph-2014) by the GRAALL group.5 Decreasing chemotherapy intensity likewise does not apply to CNS-directed prophylaxis, which may benefit from an increase in the number of intrathecal chemotherapy cycles, although an optimal regimen remains to be defined.6
The question of which TKI is the best has been the subject of intense debate and is not quite obsolete despite the results of the recent randomised PhALLCON trial, which showed superior molecular responses of the to-date most potent TKI ponatinib compared with the first-generation TKI imatinib, although overall survival was not significantly different.7 In many countries, ponatinib or even the second-generation TKI dasatinib and nilotinib are not available as first-line therapy, making equitable access to these drugs an important global endeavour. This is underscored by recent real-world evidence from a middle-income country, illustrating the need for further improvement outside of the clinical trial setting.8 Additionally, the differential side effect profiles of the various TKI´s may determine the initial choice of TKI: in case of ponatinib and nilotinib, their cardiovascular risk profile requires particular consideration in patients at risk of myocardial infarction or peripheral or arterial vascular events. This risk has been reported to be alleviated by reducing the TKI dose, but systematic long-term observations are still limited.9
A still unresolved issue is the optimal use of TKI as maintenance after HCT. Most experience has been gathered with imatinib, but all approved TKI can be safely administered. Second- and third-generation TKI are preferable in patients with more advanced and high-risk disease; in the absence of randomised data, recommendations on the duration after HCT range from 1 to 5 years of TKI following HCT.10-12 In reality, prolonged administration may prove challenging in the face of low-grade but long-term toxicities. If tolerability is poor, serial assessment of measurable residual disease and the initial relapse risk can help motivate the patient and guide post-transplant maintenance therapy. If supervised closely, pre-emptive, MRD-triggered use of TKI has been shown to be as effective as its prophylactic use in a randomised trial.13
Another emerging question concerns the potential for safely discontinuing TKI in patients who have not undergone HCT.14 As parameters predictive of the likely success of such an attempt are speculative, attempts to emulate the experience with treatment-free remissions in chronic myeloid leukaemia (CML) should be done exclusively in the setting of a clinical trial outside of a post-allogeneic transplant setting.
The improvements achieved with TKI plus chemotherapy combinations have spawned a vigorous debate about the continued role HCT, which traditionally was an almost mandatory component of post-remission therapy for Ph+ ALL. The majority of clinical trials showed superiority of strategies based on allogeneic HCT in the context of TKI plus chemotherapy regimens, and the combination of ponatinib with pediatric-inspired chemotherapy followed by alloHCT has yielded excellent outcomes,15 experience that will be relevant when the newer immunotherapeutic approaches discussed below are not available. Nevertheless, a growing body of data has identified patient subsets, defined by good molecular responses, who did not appear to benefit from transplant.16,17 In this context, the importance of assay sensitivity as a determinant of predictive power of MRD has become increasingly apparent.18,19 The major limitation of available evidence is the lack of comparability of trials in terms of molecular response, due to variability of methods for assessing MRD, their sensitivity, thresholds used for clinical decision making, time points of the analysis and therapeutic context including transplant modalities. In addition, few studies have considered additional clinical and genetic risk factors in their analysis of outcomes with or without HCT. Notably, the presence of additional recurring gene deletions, e.g. of IKZF1, CDKN2, PAX5 and others has been shown to have a profound impact on survival of Ph+ ALL patients, even among good molecular responders.20,21 Whether the negative prognostic impact of these factors will be mitigated by the newer immunotherapy approaches remains to be determined.
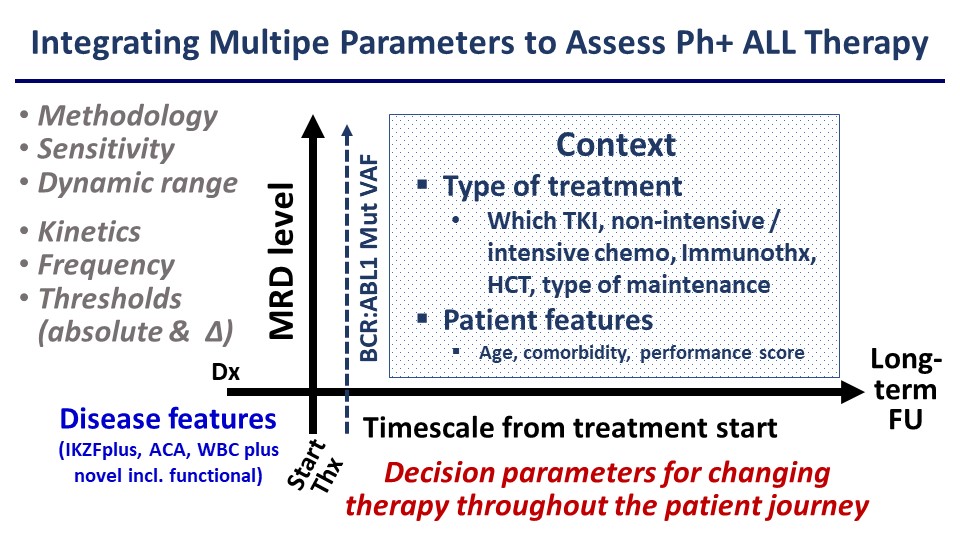
Based on this experience it would be desirable to develop more comprehensive algorithms for risk assessment rather than relying on rather simplistic approaches to MRD assessment (Figure). This should be facilitated by more sensitive and standardisable MRD quantitation by next-generation sequencing NGS and increasing availability of whole exome or whole genome sequencing and RNA-Seq. Ideally, these molecular studies would be implemented prospectively and across large cooperative study groups, encompassing both adult and paediatric patient cohorts to improve our understanding of the different disease biology between these groups. Clearly, MRD analysis and molecular stratification are central to the management not only of Ph+ ALL but all subtypes of B- or T-cell precursor ALL.
Figure
Immunotherapy for B-cell precursor ALL: blinatumomab and Inotuzumab
The addition of antibody constructs targeting cell surface antigens to our therapeutic armamentarium have had an even greater impact on our treatment strategies for ALL than the TKI as they are not restricted to a specific molecular subtype. Their major promise is the potential for enabling chemotherapy-free (except for intrathecal CNS prophylaxis) regimens by combining targeted agents of different classes, building on their high efficacy in the face of low to moderate toxicity. By extension, it is hoped that HCT can be avoided in a majority of patients. The paradigm-changing D-ALBA trial demonstrated the feasibility and efficacy of a chemotherapy-free regimen which combined dasatinib with blinatumomab as front-line therapy for Ph+ ALL.22 In a recent update of this trial with a median follow-up of 53 months, overall and event-free survival were 80.7% and 74.1%, respectively.23 It appeared that this combination was effective in eliminating clones with kinase domain mutations that were resistant to dasatinib. While the regimen was chemotherapy-free, it is noteworthy that a substantial proportion of patients, mostly those with persistent MRD, still underwent alloHCT, which was however associated with a quite low rate of transplant-associated mortality. The ponatinib-based successor trial will determine whether results can be further improved by using ponatinib as a TKI.
Evidence that the chemotherapy-free combination of blinatumomab and ponatinib may not require a subsequent HCT comes from a trial conducted by the MDACC, in which only 2% of patients underwent alloHCT and 2-year overall survival was 89%.24 Follow-up is still short, and it is worth noting that half of the relapses occurred in the CNS, again emphasising the need to improve CNS prophylaxis in the era of `exclusively´ targeted therapies.
In Philadelphia-negative B-lineage ALL omission of chemotherapy is less straightforward since the TKI class of agents cannot be employed, with the exception of some cases of BCR::ABL1-like or early T-progenitor ALL. Accordingly, the focus in Ph negative ALL is on combining immune-oncology agents with standard or reduced-intensity chemotherapy regimens, given in various sequences. The addition of 4 cycles of blinatumomab to standard consolidation chemotherapy was superior to chemotherapy consolidation alone in a randomised phase 3 trial of newly diagnosed patients with BCR::ABL1-negative ALL,25 providing the first evidence that blinatumomab significantly improved survival for both MRD negative and MRD positive patients in CR1. More recent trials are evaluating strategies incorporating both inotuzumab and blinatumomab in established front-line chemotherapy regimens such as the HyperCVAD. Initial reports suggest excellent hematologic and molecular response rates and low early mortality, but do not conclusively address the role of HCT as a definite postremission therapy. This important question needs to be addressed in a prospective randomised fashion, with patient stratification by standardised MRD and molecular analyses. One of the challenges of such a trial is the constantly evolving therapeutic landscape, in which new drugs and cellular therapies will continue to enter the clinical testing stage and if effective will prove to be significant confounding factors. At present, younger and fit patients without a good molecular response and certain patient subsets including Ph-like/BCR::ABL1-like ALL, KMT2A rearranged ALL and a hypodiploid karyotype should be considered for alloHCT.
Minimising toxicity by reducing chemotherapy intensity is particularly relevant in elderly and frail patients and is being explored by several centres and cooperative study groups. Inotuzumab alone or with low-dose chemotherapy, followed by chemotherapy, blinatumomab or both has been effective at inducing CR rates of 80-90%, mostly MRD negative, with a low induction mortality of less than 5%. Again, the importance of intrathecal chemotherapy as CNS prophylaxis cannot be overemphasised. Whether systemic chemotherapy can be completely omitted by administering inotuzumab plus blinatumumab-based regimens is unknown and is being explored primarily in elderly patients deemed ineligible for chemotherapy. Encouraging results in patients 70 years and older were obtained in an MDACC trial combining inotuzumab, blinatumomab and rituximab, although follow-up is still too short to assess long-term outcomes. Similarly, the Alliance A 041703 trial is evaluating an inotuzumab induction followed by inotuzumab and blinatumomab maintenance in patients ≥60 years.26 At the time of reporting, 9 of 33 patients had relapsed, highlighting not only the substantial challenges in treating elderly ALL patients but also the ability of ALL to evade multi-pronged targeted therapies. Accordingly, alloHCT with reduced intensity conditioning may be an option even for elderly patients who are deemed fit enough for the procedure or become fit enough after achieving a CR with low-toxicity salvage therapy.
Relapsed and refractory ALL
Treatment of patients with recurrent or resistant (r/r) disease remains the probably greatest challenge in the management of ALL. Combinations of inotuzumab, blinatumomab and chemotherapy have improved outcomes compared with historical experience, reaching approximately 40% at three years. A caveat is that as immunotherapy increasingly takes the role of standard front-line therapy, relapses will no longer respond as well to these same agents, an observation already made with sequential generations of TKI in r/r Ph+ ALL. Introduction of newer agents such as BH3 mimetics (venetoclax), menin inhibitors for KMT2A rearranged ALL, and the allosteric BCR::ABL1 inhibitor asciminib for Ph+ ALL show promise in the salvage setting, their main utility is likely to be as early front-line therapy. Transplantation is generally regarded as the definite treatment option for patients with r/r disease who achieve complete remission with salvage therapy, despite some immunotherapy-based studies showing little additional impact of HCT.
CAR T-cells targeting CD19 have emerged as a new promising therapeutic modality for advanced ALL, and two products have been approved for B-ALL in secondrelapse or in first relapse after HSCT in patients younger than 26 years (Tisegenlecleucel) and for r/r B-ALL in general (Brexucabtagene autoleucel). MRD-negative responses have been observed in approximately 60-90% of pediatric, AYA and adult patients with r/r ALL,27-31 but the median duration of response has been just over one year.30,31 This raises the question of what the optimal sequencing of available salvage therapies for patients with R/R B-ALL is and whether HSCT following CAR T-cell therapy provides long-term benefits.32 The type of prior therapies impacts on outcomes, patients who had previously received blinatumomab, inotuzumab or HCT experienced inferior survival. Disease biology as evidenced by time from HCT to relapse (< 6 months vs. ≥6 months) was also highly predictive of outcome after CAR T-cell therapy,33 as was tumour burden, disease kinetics, CAR T-cell persistence and fitness.25, 31. These factors have implications for the positioning of CAR T-cell therapy within the overall treatment strategy and raise the issue of whether HCT should be used to consolidate a CR achieved by CAR T-cells. While it was shown that CD19.28z CAR T-cells followed by a consolidative alloHSCT can provide long-term durable disease control in children and young adults with r/r B-ALL, there is no conclusive evidence for a benefit of HCT after CART therapy. In view of these challenges, current strategies to improve CAR T-cell therapy efficacy focus on multispecific CAR T-cells to overcome immune escape and new CAR designs.35-37 These exciting developments should not however obscure the fact that the cost of HCT and CAR T-cell therapy and the manufacturing process of the latter remain major challenges in providing equitable access to these therapies.
CONCLUSIONS
An increasing number of patients with ALL are now being cured by combining different types of targeted agents and positioning them in the early first-line setting. The overall reliance on cytotoxic drugs is decreasing and minimization of toxicity caused by chemotherapy has become a key concept, particularly during induction. Allogeneic HCT retains its central role in the treatment of r/r ALL but immunotherapy-based regimens seem to offer little or no benefit to an increasing number of patients, especially those with a very good molecular response. While the critical importance of MRD in informing treatment decisions is universally accepted, a lack of standardization and consensus on exactly how it should be conducted remain obstacles to its optimal use. CAR T-cells are emerging as the next major, highly effective components of ALL therapy but substantial toxicity issues need to be overcome before routinely integrating them into first-line therapy. How to best position these cellular therapies within a therapeutic regimen, including in relation to HCT, remains investigational. Treatment of older patients continues to be problematic, a better understanding of the different biology of ALL in young and older patients should be a focus of translational research. The main goal of treatment has to be the prevention of overt relapse at all costs, facilitated by optimal front-line therapy, risk-orientated stratification and meticulous implementation of state-of-the-art MRD monitoring. Even with the dramatically improved tools now at our disposal, optimal patient management requires enormous attention to detail and recognition of patient-specific parameters such as response depth and dynamics, tolerability of treatment and quality of life, psychological factors and social support structures, among others. All of these are best delivered in the context of a clinical trial.
CONFLICT OF INTEREST
Research support and honoraria for advisory board activity: Incyte, Amgen. Honoraria for advisory board activity: Autolus.
FUNDING
None
ACKNOWLEDGEMENTS
A great thanks to all colleagues, trial and nursing staff worldwide who contributed to the outstanding progress made in the treatment of ALL, and to patients and caregivers who are central to this effort.
REFERENCES
- Gokbuget N, Boissel N, Chiaretti S, et al. Management of ALL in Adults: 2023 ELN Recommendations from a European Expert Panel. Blood. 2024;10.1182/blood.2023023568
- Rousselot P, Coude MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-82. 10.1182/blood-2016-02-700153
- Short NJ, Kantarjian H, Jabbour E. SOHO State of the Art Updates & Next Questions: Intensive and Non-Intensive Approaches for Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(2):61-66. 10.1016/j.clml.2021.08.003
- Lennmyr E, Karlsson K, Ahlberg L, et al. Survival in adult acute lymphoblastic leukaemia (ALL): A report from the Swedish ALL Registry. Eur J Haematol. 2019;103(2):88-98. 10.1111/ejh.13247
- Chalandon Y, Rousselot P, Chevret S, et al. Nilotinib with or without cytarabine for Philadelphia positive acute lymphoblastic leukemia. Blood. 2024;10.1182/blood.2023023502
- Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood. 2023;141(12):1379-1388. 10.1182/blood.2022017035
- Elias Jabbour HMK, Ibrahim Aldoss, Pau Montesinos, Jessica Taft Leonard, David Gomez-Almaguer, Maria R. Baer, Carlo Gambacorti-Passerini, James McCloskey, Yosuke Minami, Cristina Papayannidis, Vanderson Geraldo Rocha, Philippe Rousselot, Pankit Vachhani, Eunice S. Wang, Bingxia Wang, Meliessa Hennessy, Alexander Vorog, Niti Patel, and Josep-Maria Ribera. First report of PhALLCON: A phase 3 study comparing ponatinib (pon) vs imatinib (im) in newly diagnosed patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Journal of Clinical Oncology. 2023;41(36)(suppl.398868). https://doi.org/10.1200/JCO.2023.41.36_suppl.398868
- Silva WF, Silverio A, Duarte BKL, et al. Philadelphia-positive B-lymphoblastic leukemia in a middle-income country - A real-world multicenter cohort. Leuk Res. 2021;110:106666. 10.1016/j.leukres.2021.106666
- Short NJ, Kantarjian H, Jabbour E, Ravandi F. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2017;30(3):193-200. 10.1016/j.beha.2017.05.001
- Nakasone H, Kako S, Mori T, et al. Stopping tyrosine kinase inhibitors started after allogeneic HCT in patients with Philadelphia chromosome-positive leukemia. Bone Marrow Transplant. 2021;56(6):1402-1412. 10.1038/s41409-020-01206-5
- Saini N, Marin D, Ledesma C, et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 8 2020;136(15):1786-1789. 10.1182/blood.2019004685
- Warraich Z, Tenneti P, Thai T, et al. Relapse Prevention with Tyrosine Kinase Inhibitors after Allogeneic Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblast Leukemia: A Systematic Review. Biol Blood Marrow Transplant. Mar 2020;26(3):e55-e64. 10.1016/j.bbmt.2019.09.022
- Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-62. 10.1038/leu.2012.352
- Samra B, Kantarjian HM, Sasaki K, et al. Discontinuation of Maintenance Tyrosine Kinase Inhibitors in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia outside of Transplant. Acta Haematol. 2021;144(3):285-292. 10.1159/000510112
- Ribera JM, Garcia-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(18):5395-5402. 10.1182/bloodadvances.2022007764
- Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-9. 10.1182/blood-2015-02-627935
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the Art Update and Next Questions: Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(7):439-446. 10.1016/j.clml.2018.05.015
- Pulsipher MA, Han X, Maude SL, et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022;3(1):66-81. 10.1158/2643-3230.BCD-21-0095
- Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196-1203. 10.1002/ajh.26949
- Hohtari H, Pallisgaard N, Kankainen M, et al. Copy number alterations define outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2022;107(8):1971-1976. 10.3324/haematol.2021.280578
- Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood. 2018;131(13):1464-1475. 10.1182/blood-2017-07-796862
- Foa R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613-1623. 10.1056/NEJMoa2016272
- Foa R, Bassan R, Elia L, et al. Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL. J Clin Oncol. 2024;42(8):881-885. 10.1200/JCO.23.01075
- Short N, Jabour E, Jain N, Huang X, Macaron W, et al. A chemotherapy-free combination of ponatinib and blinatumomab for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Hemasphere. 2023;7(Suppl):e4968152. doi: 10.1097/01.HS9.0000967384.49681.52
- Litzow MR, Sun Z, Paietta E, Mattison RJ, Lazarus HM, et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood. 2022;140(Supplement 2):LBA-1. doi: https://doi.org/10.1182/blood-2022-171751
- Wieduwilt M, Yin J, Kour O, Teske R, Stock W, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive B-cell acute lymphoblastic leukemia: ALLIANCE A041703. Hemasphere. 2023;7(Suppl):e08838b7. doi: 10.1097/01.HS9.0000967380.08838.b7
- Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. 10.1182/blood-2017-02-769208
- Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652-1663. 10.1182/blood-2018-11-883710
- Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449-459. 10.1056/NEJMoa1709919
- Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. 10.1182/bloodadvances.2020003092
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 10.1016/S0140-6736(21)01222-8
- Shah BD, Cassaday RD, Park JH, et al. Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3. J Immunother Cancer. 2023;11(8)10.1136/jitc-2023-007118
- Bader P, Rossig C, Hutter M, et al. CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv. 2023;7(11):2436-2448. 10.1182/bloodadvances.2022008981
- Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(27):3044-3055. 10.1200/JCO.20.03458
- Benjamin R, Graham C, Yallop D, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396(10266):1885-1894. 10.1016/S0140-6736(20)32334-5
- Roddie C, Dias J, O'Reilly MA, et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(30):3352-3363. 10.1200/JCO.21.00917
- Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419-1431. 10.1038/s41591-021-01436-0
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)?
Next Article
Definitions of acute myeloid leukaemia and their clinical significance according to the WHO 2022 and ICC classification »
Related Articles
April 30, 2024
Acute myeloid leukaemia: First debate on leukaemia biology
© 2024 Medicom Medical Publishers. All rights reserved.
Terms and Conditions
| Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com
ABL1-directed TKI were the first class of targeted agents that proved to be game changers; successive generations of TKI were able to induce – as single agents or in combination with corticosteroids - a complete remission in nearly all patients diagnosed with Ph+ ALL. This, plus a substantially lower toxicity and early death rate with improved outcomes facilitated reduced-intensity induction treatment as a new therapeutic principle.1-3 Moreover, the use of TKI in combination with established post-remission regimens (chemotherapy and HCT) resulted in survival outcomes equivalent to those of patients with Philadelphia-negative ALL. 4
As a caveat, the concept of using TKI alone in combination with substantially reduced intensity chemotherapy applies to induction therapy for Ph+ ALL, but not to consolidation cycles, at least in the absence of immunotherapy. This was demonstrated by an increased relapse rate in patients in whom high-dose cytarabine was omitted from consolidation therapy in a recent randomised, nilotinib-based trial (Graaph-2014) by the GRAALL group.5 Decreasing chemotherapy intensity likewise does not apply to CNS-directed prophylaxis, which may benefit from an increase in the number of intrathecal chemotherapy cycles, although an optimal regimen remains to be defined.6
The question of which TKI is the best has been the subject of intense debate and is not quite obsolete despite the results of the recent randomised PhALLCON trial, which showed superior molecular responses of the to-date most potent TKI ponatinib compared with the first-generation TKI imatinib, although overall survival was not significantly different.7 In many countries, ponatinib or even the second-generation TKI dasatinib and nilotinib are not available as first-line therapy, making equitable access to these drugs an important global endeavour. This is underscored by recent real-world evidence from a middle-income country, illustrating the need for further improvement outside of the clinical trial setting.8 Additionally, the differential side effect profiles of the various TKI´s may determine the initial choice of TKI: in case of ponatinib and nilotinib, their cardiovascular risk profile requires particular consideration in patients at risk of myocardial infarction or peripheral or arterial vascular events. This risk has been reported to be alleviated by reducing the TKI dose, but systematic long-term observations are still limited.9
A still unresolved issue is the optimal use of TKI as maintenance after HCT. Most experience has been gathered with imatinib, but all approved TKI can be safely administered. Second- and third-generation TKI are preferable in patients with more advanced and high-risk disease; in the absence of randomised data, recommendations on the duration after HCT range from 1 to 5 years of TKI following HCT.10-12 In reality, prolonged administration may prove challenging in the face of low-grade but long-term toxicities. If tolerability is poor, serial assessment of measurable residual disease and the initial relapse risk can help motivate the patient and guide post-transplant maintenance therapy. If supervised closely, pre-emptive, MRD-triggered use of TKI has been shown to be as effective as its prophylactic use in a randomised trial.13
Another emerging question concerns the potential for safely discontinuing TKI in patients who have not undergone HCT.14 As parameters predictive of the likely success of such an attempt are speculative, attempts to emulate the experience with treatment-free remissions in chronic myeloid leukaemia (CML) should be done exclusively in the setting of a clinical trial outside of a post-allogeneic transplant setting.
The improvements achieved with TKI plus chemotherapy combinations have spawned a vigorous debate about the continued role HCT, which traditionally was an almost mandatory component of post-remission therapy for Ph+ ALL. The majority of clinical trials showed superiority of strategies based on allogeneic HCT in the context of TKI plus chemotherapy regimens, and the combination of ponatinib with pediatric-inspired chemotherapy followed by alloHCT has yielded excellent outcomes,15 experience that will be relevant when the newer immunotherapeutic approaches discussed below are not available. Nevertheless, a growing body of data has identified patient subsets, defined by good molecular responses, who did not appear to benefit from transplant.16,17 In this context, the importance of assay sensitivity as a determinant of predictive power of MRD has become increasingly apparent.18,19 The major limitation of available evidence is the lack of comparability of trials in terms of molecular response, due to variability of methods for assessing MRD, their sensitivity, thresholds used for clinical decision making, time points of the analysis and therapeutic context including transplant modalities. In addition, few studies have considered additional clinical and genetic risk factors in their analysis of outcomes with or without HCT. Notably, the presence of additional recurring gene deletions, e.g. of IKZF1, CDKN2, PAX5 and others has been shown to have a profound impact on survival of Ph+ ALL patients, even among good molecular responders.20,21 Whether the negative prognostic impact of these factors will be mitigated by the newer immunotherapy approaches remains to be determined.
Based on this experience it would be desirable to develop more comprehensive algorithms for risk assessment rather than relying on rather simplistic approaches to MRD assessment (Figure). This should be facilitated by more sensitive and standardisable MRD quantitation by next-generation sequencing NGS and increasing availability of whole exome or whole genome sequencing and RNA-Seq. Ideally, these molecular studies would be implemented prospectively and across large cooperative study groups, encompassing both adult and paediatric patient cohorts to improve our understanding of the different disease biology between these groups. Clearly, MRD analysis and molecular stratification are central to the management not only of Ph+ ALL but all subtypes of B- or T-cell precursor ALL.
Figure

Immunotherapy for B-cell precursor ALL: blinatumomab and Inotuzumab
The addition of antibody constructs targeting cell surface antigens to our therapeutic armamentarium have had an even greater impact on our treatment strategies for ALL than the TKI as they are not restricted to a specific molecular subtype. Their major promise is the potential for enabling chemotherapy-free (except for intrathecal CNS prophylaxis) regimens by combining targeted agents of different classes, building on their high efficacy in the face of low to moderate toxicity. By extension, it is hoped that HCT can be avoided in a majority of patients. The paradigm-changing D-ALBA trial demonstrated the feasibility and efficacy of a chemotherapy-free regimen which combined dasatinib with blinatumomab as front-line therapy for Ph+ ALL.22 In a recent update of this trial with a median follow-up of 53 months, overall and event-free survival were 80.7% and 74.1%, respectively.23 It appeared that this combination was effective in eliminating clones with kinase domain mutations that were resistant to dasatinib. While the regimen was chemotherapy-free, it is noteworthy that a substantial proportion of patients, mostly those with persistent MRD, still underwent alloHCT, which was however associated with a quite low rate of transplant-associated mortality. The ponatinib-based successor trial will determine whether results can be further improved by using ponatinib as a TKI.
Evidence that the chemotherapy-free combination of blinatumomab and ponatinib may not require a subsequent HCT comes from a trial conducted by the MDACC, in which only 2% of patients underwent alloHCT and 2-year overall survival was 89%.24 Follow-up is still short, and it is worth noting that half of the relapses occurred in the CNS, again emphasising the need to improve CNS prophylaxis in the era of `exclusively´ targeted therapies.
In Philadelphia-negative B-lineage ALL omission of chemotherapy is less straightforward since the TKI class of agents cannot be employed, with the exception of some cases of BCR::ABL1-like or early T-progenitor ALL. Accordingly, the focus in Ph negative ALL is on combining immune-oncology agents with standard or reduced-intensity chemotherapy regimens, given in various sequences. The addition of 4 cycles of blinatumomab to standard consolidation chemotherapy was superior to chemotherapy consolidation alone in a randomised phase 3 trial of newly diagnosed patients with BCR::ABL1-negative ALL,25 providing the first evidence that blinatumomab significantly improved survival for both MRD negative and MRD positive patients in CR1. More recent trials are evaluating strategies incorporating both inotuzumab and blinatumomab in established front-line chemotherapy regimens such as the HyperCVAD. Initial reports suggest excellent hematologic and molecular response rates and low early mortality, but do not conclusively address the role of HCT as a definite postremission therapy. This important question needs to be addressed in a prospective randomised fashion, with patient stratification by standardised MRD and molecular analyses. One of the challenges of such a trial is the constantly evolving therapeutic landscape, in which new drugs and cellular therapies will continue to enter the clinical testing stage and if effective will prove to be significant confounding factors. At present, younger and fit patients without a good molecular response and certain patient subsets including Ph-like/BCR::ABL1-like ALL, KMT2A rearranged ALL and a hypodiploid karyotype should be considered for alloHCT.
Minimising toxicity by reducing chemotherapy intensity is particularly relevant in elderly and frail patients and is being explored by several centres and cooperative study groups. Inotuzumab alone or with low-dose chemotherapy, followed by chemotherapy, blinatumomab or both has been effective at inducing CR rates of 80-90%, mostly MRD negative, with a low induction mortality of less than 5%. Again, the importance of intrathecal chemotherapy as CNS prophylaxis cannot be overemphasised. Whether systemic chemotherapy can be completely omitted by administering inotuzumab plus blinatumumab-based regimens is unknown and is being explored primarily in elderly patients deemed ineligible for chemotherapy. Encouraging results in patients 70 years and older were obtained in an MDACC trial combining inotuzumab, blinatumomab and rituximab, although follow-up is still too short to assess long-term outcomes. Similarly, the Alliance A 041703 trial is evaluating an inotuzumab induction followed by inotuzumab and blinatumomab maintenance in patients ≥60 years.26 At the time of reporting, 9 of 33 patients had relapsed, highlighting not only the substantial challenges in treating elderly ALL patients but also the ability of ALL to evade multi-pronged targeted therapies. Accordingly, alloHCT with reduced intensity conditioning may be an option even for elderly patients who are deemed fit enough for the procedure or become fit enough after achieving a CR with low-toxicity salvage therapy.
Relapsed and refractory ALL
Treatment of patients with recurrent or resistant (r/r) disease remains the probably greatest challenge in the management of ALL. Combinations of inotuzumab, blinatumomab and chemotherapy have improved outcomes compared with historical experience, reaching approximately 40% at three years. A caveat is that as immunotherapy increasingly takes the role of standard front-line therapy, relapses will no longer respond as well to these same agents, an observation already made with sequential generations of TKI in r/r Ph+ ALL. Introduction of newer agents such as BH3 mimetics (venetoclax), menin inhibitors for KMT2A rearranged ALL, and the allosteric BCR::ABL1 inhibitor asciminib for Ph+ ALL show promise in the salvage setting, their main utility is likely to be as early front-line therapy. Transplantation is generally regarded as the definite treatment option for patients with r/r disease who achieve complete remission with salvage therapy, despite some immunotherapy-based studies showing little additional impact of HCT.
CAR T-cells targeting CD19 have emerged as a new promising therapeutic modality for advanced ALL, and two products have been approved for B-ALL in secondrelapse or in first relapse after HSCT in patients younger than 26 years (Tisegenlecleucel) and for r/r B-ALL in general (Brexucabtagene autoleucel). MRD-negative responses have been observed in approximately 60-90% of pediatric, AYA and adult patients with r/r ALL,27-31 but the median duration of response has been just over one year.30,31 This raises the question of what the optimal sequencing of available salvage therapies for patients with R/R B-ALL is and whether HSCT following CAR T-cell therapy provides long-term benefits.32 The type of prior therapies impacts on outcomes, patients who had previously received blinatumomab, inotuzumab or HCT experienced inferior survival. Disease biology as evidenced by time from HCT to relapse (< 6 months vs. ≥6 months) was also highly predictive of outcome after CAR T-cell therapy,33 as was tumour burden, disease kinetics, CAR T-cell persistence and fitness.25, 31. These factors have implications for the positioning of CAR T-cell therapy within the overall treatment strategy and raise the issue of whether HCT should be used to consolidate a CR achieved by CAR T-cells. While it was shown that CD19.28z CAR T-cells followed by a consolidative alloHSCT can provide long-term durable disease control in children and young adults with r/r B-ALL, there is no conclusive evidence for a benefit of HCT after CART therapy. In view of these challenges, current strategies to improve CAR T-cell therapy efficacy focus on multispecific CAR T-cells to overcome immune escape and new CAR designs.35-37 These exciting developments should not however obscure the fact that the cost of HCT and CAR T-cell therapy and the manufacturing process of the latter remain major challenges in providing equitable access to these therapies.
CONCLUSIONS
An increasing number of patients with ALL are now being cured by combining different types of targeted agents and positioning them in the early first-line setting. The overall reliance on cytotoxic drugs is decreasing and minimization of toxicity caused by chemotherapy has become a key concept, particularly during induction. Allogeneic HCT retains its central role in the treatment of r/r ALL but immunotherapy-based regimens seem to offer little or no benefit to an increasing number of patients, especially those with a very good molecular response. While the critical importance of MRD in informing treatment decisions is universally accepted, a lack of standardization and consensus on exactly how it should be conducted remain obstacles to its optimal use. CAR T-cells are emerging as the next major, highly effective components of ALL therapy but substantial toxicity issues need to be overcome before routinely integrating them into first-line therapy. How to best position these cellular therapies within a therapeutic regimen, including in relation to HCT, remains investigational. Treatment of older patients continues to be problematic, a better understanding of the different biology of ALL in young and older patients should be a focus of translational research. The main goal of treatment has to be the prevention of overt relapse at all costs, facilitated by optimal front-line therapy, risk-orientated stratification and meticulous implementation of state-of-the-art MRD monitoring. Even with the dramatically improved tools now at our disposal, optimal patient management requires enormous attention to detail and recognition of patient-specific parameters such as response depth and dynamics, tolerability of treatment and quality of life, psychological factors and social support structures, among others. All of these are best delivered in the context of a clinical trial.
CONFLICT OF INTEREST
Research support and honoraria for advisory board activity: Incyte, Amgen. Honoraria for advisory board activity: Autolus.
FUNDING
None
ACKNOWLEDGEMENTS
A great thanks to all colleagues, trial and nursing staff worldwide who contributed to the outstanding progress made in the treatment of ALL, and to patients and caregivers who are central to this effort.
REFERENCES
- Gokbuget N, Boissel N, Chiaretti S, et al. Management of ALL in Adults: 2023 ELN Recommendations from a European Expert Panel. Blood. 2024;10.1182/blood.2023023568
- Rousselot P, Coude MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-82. 10.1182/blood-2016-02-700153
- Short NJ, Kantarjian H, Jabbour E. SOHO State of the Art Updates & Next Questions: Intensive and Non-Intensive Approaches for Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(2):61-66. 10.1016/j.clml.2021.08.003
- Lennmyr E, Karlsson K, Ahlberg L, et al. Survival in adult acute lymphoblastic leukaemia (ALL): A report from the Swedish ALL Registry. Eur J Haematol. 2019;103(2):88-98. 10.1111/ejh.13247
- Chalandon Y, Rousselot P, Chevret S, et al. Nilotinib with or without cytarabine for Philadelphia positive acute lymphoblastic leukemia. Blood. 2024;10.1182/blood.2023023502
- Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood. 2023;141(12):1379-1388. 10.1182/blood.2022017035
- Elias Jabbour HMK, Ibrahim Aldoss, Pau Montesinos, Jessica Taft Leonard, David Gomez-Almaguer, Maria R. Baer, Carlo Gambacorti-Passerini, James McCloskey, Yosuke Minami, Cristina Papayannidis, Vanderson Geraldo Rocha, Philippe Rousselot, Pankit Vachhani, Eunice S. Wang, Bingxia Wang, Meliessa Hennessy, Alexander Vorog, Niti Patel, and Josep-Maria Ribera. First report of PhALLCON: A phase 3 study comparing ponatinib (pon) vs imatinib (im) in newly diagnosed patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Journal of Clinical Oncology. 2023;41(36)(suppl.398868). https://doi.org/10.1200/JCO.2023.41.36_suppl.398868
- Silva WF, Silverio A, Duarte BKL, et al. Philadelphia-positive B-lymphoblastic leukemia in a middle-income country - A real-world multicenter cohort. Leuk Res. 2021;110:106666. 10.1016/j.leukres.2021.106666
- Short NJ, Kantarjian H, Jabbour E, Ravandi F. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2017;30(3):193-200. 10.1016/j.beha.2017.05.001
- Nakasone H, Kako S, Mori T, et al. Stopping tyrosine kinase inhibitors started after allogeneic HCT in patients with Philadelphia chromosome-positive leukemia. Bone Marrow Transplant. 2021;56(6):1402-1412. 10.1038/s41409-020-01206-5
- Saini N, Marin D, Ledesma C, et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 8 2020;136(15):1786-1789. 10.1182/blood.2019004685
- Warraich Z, Tenneti P, Thai T, et al. Relapse Prevention with Tyrosine Kinase Inhibitors after Allogeneic Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblast Leukemia: A Systematic Review. Biol Blood Marrow Transplant. Mar 2020;26(3):e55-e64. 10.1016/j.bbmt.2019.09.022
- Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-62. 10.1038/leu.2012.352
- Samra B, Kantarjian HM, Sasaki K, et al. Discontinuation of Maintenance Tyrosine Kinase Inhibitors in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia outside of Transplant. Acta Haematol. 2021;144(3):285-292. 10.1159/000510112
- Ribera JM, Garcia-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(18):5395-5402. 10.1182/bloodadvances.2022007764
- Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-9. 10.1182/blood-2015-02-627935
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the Art Update and Next Questions: Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(7):439-446. 10.1016/j.clml.2018.05.015
- Pulsipher MA, Han X, Maude SL, et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022;3(1):66-81. 10.1158/2643-3230.BCD-21-0095
- Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196-1203. 10.1002/ajh.26949
- Hohtari H, Pallisgaard N, Kankainen M, et al. Copy number alterations define outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2022;107(8):1971-1976. 10.3324/haematol.2021.280578
- Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood. 2018;131(13):1464-1475. 10.1182/blood-2017-07-796862
- Foa R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613-1623. 10.1056/NEJMoa2016272
- Foa R, Bassan R, Elia L, et al. Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL. J Clin Oncol. 2024;42(8):881-885. 10.1200/JCO.23.01075
- Short N, Jabour E, Jain N, Huang X, Macaron W, et al. A chemotherapy-free combination of ponatinib and blinatumomab for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Hemasphere. 2023;7(Suppl):e4968152. doi: 10.1097/01.HS9.0000967384.49681.52
- Litzow MR, Sun Z, Paietta E, Mattison RJ, Lazarus HM, et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood. 2022;140(Supplement 2):LBA-1. doi: https://doi.org/10.1182/blood-2022-171751
- Wieduwilt M, Yin J, Kour O, Teske R, Stock W, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive B-cell acute lymphoblastic leukemia: ALLIANCE A041703. Hemasphere. 2023;7(Suppl):e08838b7. doi: 10.1097/01.HS9.0000967380.08838.b7
- Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. 10.1182/blood-2017-02-769208
- Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652-1663. 10.1182/blood-2018-11-883710
- Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449-459. 10.1056/NEJMoa1709919
- Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. 10.1182/bloodadvances.2020003092
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 10.1016/S0140-6736(21)01222-8
- Shah BD, Cassaday RD, Park JH, et al. Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3. J Immunother Cancer. 2023;11(8)10.1136/jitc-2023-007118
- Bader P, Rossig C, Hutter M, et al. CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv. 2023;7(11):2436-2448. 10.1182/bloodadvances.2022008981
- Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(27):3044-3055. 10.1200/JCO.20.03458
- Benjamin R, Graham C, Yallop D, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396(10266):1885-1894. 10.1016/S0140-6736(20)32334-5
- Roddie C, Dias J, O'Reilly MA, et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(30):3352-3363. 10.1200/JCO.21.00917
- Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419-1431. 10.1038/s41591-021-01436-0
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)?
Next Article
Definitions of acute myeloid leukaemia and their clinical significance according to the WHO 2022 and ICC classification »
Related Articles
April 30, 2024
Acute myeloid leukaemia: First debate on leukaemia biology
© 2024 Medicom Medical Publishers. All rights reserved.
Terms and Conditions
| Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com
Treatment of patients with recurrent or resistant (r/r) disease remains the probably greatest challenge in the management of ALL. Combinations of inotuzumab, blinatumomab and chemotherapy have improved outcomes compared with historical experience, reaching approximately 40% at three years. A caveat is that as immunotherapy increasingly takes the role of standard front-line therapy, relapses will no longer respond as well to these same agents, an observation already made with sequential generations of TKI in r/r Ph+ ALL. Introduction of newer agents such as BH3 mimetics (venetoclax), menin inhibitors for KMT2A rearranged ALL, and the allosteric BCR::ABL1 inhibitor asciminib for Ph+ ALL show promise in the salvage setting, their main utility is likely to be as early front-line therapy. Transplantation is generally regarded as the definite treatment option for patients with r/r disease who achieve complete remission with salvage therapy, despite some immunotherapy-based studies showing little additional impact of HCT.
CAR T-cells targeting CD19 have emerged as a new promising therapeutic modality for advanced ALL, and two products have been approved for B-ALL in secondrelapse or in first relapse after HSCT in patients younger than 26 years (Tisegenlecleucel) and for r/r B-ALL in general (Brexucabtagene autoleucel). MRD-negative responses have been observed in approximately 60-90% of pediatric, AYA and adult patients with r/r ALL,27-31 but the median duration of response has been just over one year.30,31 This raises the question of what the optimal sequencing of available salvage therapies for patients with R/R B-ALL is and whether HSCT following CAR T-cell therapy provides long-term benefits.32 The type of prior therapies impacts on outcomes, patients who had previously received blinatumomab, inotuzumab or HCT experienced inferior survival. Disease biology as evidenced by time from HCT to relapse (< 6 months vs. ≥6 months) was also highly predictive of outcome after CAR T-cell therapy,33 as was tumour burden, disease kinetics, CAR T-cell persistence and fitness.25, 31. These factors have implications for the positioning of CAR T-cell therapy within the overall treatment strategy and raise the issue of whether HCT should be used to consolidate a CR achieved by CAR T-cells. While it was shown that CD19.28z CAR T-cells followed by a consolidative alloHSCT can provide long-term durable disease control in children and young adults with r/r B-ALL, there is no conclusive evidence for a benefit of HCT after CART therapy. In view of these challenges, current strategies to improve CAR T-cell therapy efficacy focus on multispecific CAR T-cells to overcome immune escape and new CAR designs.35-37 These exciting developments should not however obscure the fact that the cost of HCT and CAR T-cell therapy and the manufacturing process of the latter remain major challenges in providing equitable access to these therapies.
CONCLUSIONS
An increasing number of patients with ALL are now being cured by combining different types of targeted agents and positioning them in the early first-line setting. The overall reliance on cytotoxic drugs is decreasing and minimization of toxicity caused by chemotherapy has become a key concept, particularly during induction. Allogeneic HCT retains its central role in the treatment of r/r ALL but immunotherapy-based regimens seem to offer little or no benefit to an increasing number of patients, especially those with a very good molecular response. While the critical importance of MRD in informing treatment decisions is universally accepted, a lack of standardization and consensus on exactly how it should be conducted remain obstacles to its optimal use. CAR T-cells are emerging as the next major, highly effective components of ALL therapy but substantial toxicity issues need to be overcome before routinely integrating them into first-line therapy. How to best position these cellular therapies within a therapeutic regimen, including in relation to HCT, remains investigational. Treatment of older patients continues to be problematic, a better understanding of the different biology of ALL in young and older patients should be a focus of translational research. The main goal of treatment has to be the prevention of overt relapse at all costs, facilitated by optimal front-line therapy, risk-orientated stratification and meticulous implementation of state-of-the-art MRD monitoring. Even with the dramatically improved tools now at our disposal, optimal patient management requires enormous attention to detail and recognition of patient-specific parameters such as response depth and dynamics, tolerability of treatment and quality of life, psychological factors and social support structures, among others. All of these are best delivered in the context of a clinical trial.
CONFLICT OF INTEREST
Research support and honoraria for advisory board activity: Incyte, Amgen. Honoraria for advisory board activity: Autolus.
FUNDING
None
ACKNOWLEDGEMENTS
A great thanks to all colleagues, trial and nursing staff worldwide who contributed to the outstanding progress made in the treatment of ALL, and to patients and caregivers who are central to this effort.
REFERENCES
- Gokbuget N, Boissel N, Chiaretti S, et al. Management of ALL in Adults: 2023 ELN Recommendations from a European Expert Panel. Blood. 2024;10.1182/blood.2023023568
- Rousselot P, Coude MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-82. 10.1182/blood-2016-02-700153
- Short NJ, Kantarjian H, Jabbour E. SOHO State of the Art Updates & Next Questions: Intensive and Non-Intensive Approaches for Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(2):61-66. 10.1016/j.clml.2021.08.003
- Lennmyr E, Karlsson K, Ahlberg L, et al. Survival in adult acute lymphoblastic leukaemia (ALL): A report from the Swedish ALL Registry. Eur J Haematol. 2019;103(2):88-98. 10.1111/ejh.13247
- Chalandon Y, Rousselot P, Chevret S, et al. Nilotinib with or without cytarabine for Philadelphia positive acute lymphoblastic leukemia. Blood. 2024;10.1182/blood.2023023502
- Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood. 2023;141(12):1379-1388. 10.1182/blood.2022017035
- Elias Jabbour HMK, Ibrahim Aldoss, Pau Montesinos, Jessica Taft Leonard, David Gomez-Almaguer, Maria R. Baer, Carlo Gambacorti-Passerini, James McCloskey, Yosuke Minami, Cristina Papayannidis, Vanderson Geraldo Rocha, Philippe Rousselot, Pankit Vachhani, Eunice S. Wang, Bingxia Wang, Meliessa Hennessy, Alexander Vorog, Niti Patel, and Josep-Maria Ribera. First report of PhALLCON: A phase 3 study comparing ponatinib (pon) vs imatinib (im) in newly diagnosed patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Journal of Clinical Oncology. 2023;41(36)(suppl.398868). https://doi.org/10.1200/JCO.2023.41.36_suppl.398868
- Silva WF, Silverio A, Duarte BKL, et al. Philadelphia-positive B-lymphoblastic leukemia in a middle-income country - A real-world multicenter cohort. Leuk Res. 2021;110:106666. 10.1016/j.leukres.2021.106666
- Short NJ, Kantarjian H, Jabbour E, Ravandi F. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2017;30(3):193-200. 10.1016/j.beha.2017.05.001
- Nakasone H, Kako S, Mori T, et al. Stopping tyrosine kinase inhibitors started after allogeneic HCT in patients with Philadelphia chromosome-positive leukemia. Bone Marrow Transplant. 2021;56(6):1402-1412. 10.1038/s41409-020-01206-5
- Saini N, Marin D, Ledesma C, et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 8 2020;136(15):1786-1789. 10.1182/blood.2019004685
- Warraich Z, Tenneti P, Thai T, et al. Relapse Prevention with Tyrosine Kinase Inhibitors after Allogeneic Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblast Leukemia: A Systematic Review. Biol Blood Marrow Transplant. Mar 2020;26(3):e55-e64. 10.1016/j.bbmt.2019.09.022
- Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-62. 10.1038/leu.2012.352
- Samra B, Kantarjian HM, Sasaki K, et al. Discontinuation of Maintenance Tyrosine Kinase Inhibitors in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia outside of Transplant. Acta Haematol. 2021;144(3):285-292. 10.1159/000510112
- Ribera JM, Garcia-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(18):5395-5402. 10.1182/bloodadvances.2022007764
- Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-9. 10.1182/blood-2015-02-627935
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the Art Update and Next Questions: Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(7):439-446. 10.1016/j.clml.2018.05.015
- Pulsipher MA, Han X, Maude SL, et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022;3(1):66-81. 10.1158/2643-3230.BCD-21-0095
- Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196-1203. 10.1002/ajh.26949
- Hohtari H, Pallisgaard N, Kankainen M, et al. Copy number alterations define outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2022;107(8):1971-1976. 10.3324/haematol.2021.280578
- Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood. 2018;131(13):1464-1475. 10.1182/blood-2017-07-796862
- Foa R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613-1623. 10.1056/NEJMoa2016272
- Foa R, Bassan R, Elia L, et al. Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL. J Clin Oncol. 2024;42(8):881-885. 10.1200/JCO.23.01075
- Short N, Jabour E, Jain N, Huang X, Macaron W, et al. A chemotherapy-free combination of ponatinib and blinatumomab for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Hemasphere. 2023;7(Suppl):e4968152. doi: 10.1097/01.HS9.0000967384.49681.52
- Litzow MR, Sun Z, Paietta E, Mattison RJ, Lazarus HM, et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood. 2022;140(Supplement 2):LBA-1. doi: https://doi.org/10.1182/blood-2022-171751
- Wieduwilt M, Yin J, Kour O, Teske R, Stock W, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive B-cell acute lymphoblastic leukemia: ALLIANCE A041703. Hemasphere. 2023;7(Suppl):e08838b7. doi: 10.1097/01.HS9.0000967380.08838.b7
- Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. 10.1182/blood-2017-02-769208
- Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652-1663. 10.1182/blood-2018-11-883710
- Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449-459. 10.1056/NEJMoa1709919
- Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. 10.1182/bloodadvances.2020003092
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 10.1016/S0140-6736(21)01222-8
- Shah BD, Cassaday RD, Park JH, et al. Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3. J Immunother Cancer. 2023;11(8)10.1136/jitc-2023-007118
- Bader P, Rossig C, Hutter M, et al. CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv. 2023;7(11):2436-2448. 10.1182/bloodadvances.2022008981
- Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(27):3044-3055. 10.1200/JCO.20.03458
- Benjamin R, Graham C, Yallop D, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396(10266):1885-1894. 10.1016/S0140-6736(20)32334-5
- Roddie C, Dias J, O'Reilly MA, et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(30):3352-3363. 10.1200/JCO.21.00917
- Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419-1431. 10.1038/s41591-021-01436-0
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)?
Next Article
Definitions of acute myeloid leukaemia and their clinical significance according to the WHO 2022 and ICC classification »
Related Articles
April 30, 2024
Acute myeloid leukaemia: First debate on leukaemia biology
© 2024 Medicom Medical Publishers. All rights reserved.
Terms and Conditions
| Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com
Research support and honoraria for advisory board activity: Incyte, Amgen. Honoraria for advisory board activity: Autolus.
FUNDING
None
ACKNOWLEDGEMENTS
A great thanks to all colleagues, trial and nursing staff worldwide who contributed to the outstanding progress made in the treatment of ALL, and to patients and caregivers who are central to this effort.
REFERENCES
- Gokbuget N, Boissel N, Chiaretti S, et al. Management of ALL in Adults: 2023 ELN Recommendations from a European Expert Panel. Blood. 2024;10.1182/blood.2023023568
- Rousselot P, Coude MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-82. 10.1182/blood-2016-02-700153
- Short NJ, Kantarjian H, Jabbour E. SOHO State of the Art Updates & Next Questions: Intensive and Non-Intensive Approaches for Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(2):61-66. 10.1016/j.clml.2021.08.003
- Lennmyr E, Karlsson K, Ahlberg L, et al. Survival in adult acute lymphoblastic leukaemia (ALL): A report from the Swedish ALL Registry. Eur J Haematol. 2019;103(2):88-98. 10.1111/ejh.13247
- Chalandon Y, Rousselot P, Chevret S, et al. Nilotinib with or without cytarabine for Philadelphia positive acute lymphoblastic leukemia. Blood. 2024;10.1182/blood.2023023502
- Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood. 2023;141(12):1379-1388. 10.1182/blood.2022017035
- Elias Jabbour HMK, Ibrahim Aldoss, Pau Montesinos, Jessica Taft Leonard, David Gomez-Almaguer, Maria R. Baer, Carlo Gambacorti-Passerini, James McCloskey, Yosuke Minami, Cristina Papayannidis, Vanderson Geraldo Rocha, Philippe Rousselot, Pankit Vachhani, Eunice S. Wang, Bingxia Wang, Meliessa Hennessy, Alexander Vorog, Niti Patel, and Josep-Maria Ribera. First report of PhALLCON: A phase 3 study comparing ponatinib (pon) vs imatinib (im) in newly diagnosed patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Journal of Clinical Oncology. 2023;41(36)(suppl.398868). https://doi.org/10.1200/JCO.2023.41.36_suppl.398868
- Silva WF, Silverio A, Duarte BKL, et al. Philadelphia-positive B-lymphoblastic leukemia in a middle-income country - A real-world multicenter cohort. Leuk Res. 2021;110:106666. 10.1016/j.leukres.2021.106666
- Short NJ, Kantarjian H, Jabbour E, Ravandi F. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2017;30(3):193-200. 10.1016/j.beha.2017.05.001
- Nakasone H, Kako S, Mori T, et al. Stopping tyrosine kinase inhibitors started after allogeneic HCT in patients with Philadelphia chromosome-positive leukemia. Bone Marrow Transplant. 2021;56(6):1402-1412. 10.1038/s41409-020-01206-5
- Saini N, Marin D, Ledesma C, et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 8 2020;136(15):1786-1789. 10.1182/blood.2019004685
- Warraich Z, Tenneti P, Thai T, et al. Relapse Prevention with Tyrosine Kinase Inhibitors after Allogeneic Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblast Leukemia: A Systematic Review. Biol Blood Marrow Transplant. Mar 2020;26(3):e55-e64. 10.1016/j.bbmt.2019.09.022
- Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-62. 10.1038/leu.2012.352
- Samra B, Kantarjian HM, Sasaki K, et al. Discontinuation of Maintenance Tyrosine Kinase Inhibitors in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia outside of Transplant. Acta Haematol. 2021;144(3):285-292. 10.1159/000510112
- Ribera JM, Garcia-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(18):5395-5402. 10.1182/bloodadvances.2022007764
- Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-9. 10.1182/blood-2015-02-627935
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the Art Update and Next Questions: Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(7):439-446. 10.1016/j.clml.2018.05.015
- Pulsipher MA, Han X, Maude SL, et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022;3(1):66-81. 10.1158/2643-3230.BCD-21-0095
- Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196-1203. 10.1002/ajh.26949
- Hohtari H, Pallisgaard N, Kankainen M, et al. Copy number alterations define outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2022;107(8):1971-1976. 10.3324/haematol.2021.280578
- Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood. 2018;131(13):1464-1475. 10.1182/blood-2017-07-796862
- Foa R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613-1623. 10.1056/NEJMoa2016272
- Foa R, Bassan R, Elia L, et al. Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL. J Clin Oncol. 2024;42(8):881-885. 10.1200/JCO.23.01075
- Short N, Jabour E, Jain N, Huang X, Macaron W, et al. A chemotherapy-free combination of ponatinib and blinatumomab for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Hemasphere. 2023;7(Suppl):e4968152. doi: 10.1097/01.HS9.0000967384.49681.52
- Litzow MR, Sun Z, Paietta E, Mattison RJ, Lazarus HM, et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood. 2022;140(Supplement 2):LBA-1. doi: https://doi.org/10.1182/blood-2022-171751
- Wieduwilt M, Yin J, Kour O, Teske R, Stock W, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive B-cell acute lymphoblastic leukemia: ALLIANCE A041703. Hemasphere. 2023;7(Suppl):e08838b7. doi: 10.1097/01.HS9.0000967380.08838.b7
- Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. 10.1182/blood-2017-02-769208
- Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652-1663. 10.1182/blood-2018-11-883710
- Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449-459. 10.1056/NEJMoa1709919
- Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. 10.1182/bloodadvances.2020003092
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 10.1016/S0140-6736(21)01222-8
- Shah BD, Cassaday RD, Park JH, et al. Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3. J Immunother Cancer. 2023;11(8)10.1136/jitc-2023-007118
- Bader P, Rossig C, Hutter M, et al. CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv. 2023;7(11):2436-2448. 10.1182/bloodadvances.2022008981
- Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(27):3044-3055. 10.1200/JCO.20.03458
- Benjamin R, Graham C, Yallop D, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396(10266):1885-1894. 10.1016/S0140-6736(20)32334-5
- Roddie C, Dias J, O'Reilly MA, et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(30):3352-3363. 10.1200/JCO.21.00917
- Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419-1431. 10.1038/s41591-021-01436-0
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)?
Next Article
Definitions of acute myeloid leukaemia and their clinical significance according to the WHO 2022 and ICC classification »
Related Articles
April 30, 2024
Acute myeloid leukaemia: First debate on leukaemia biology
© 2024 Medicom Medical Publishers. All rights reserved.
Terms and Conditions
| Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com
A great thanks to all colleagues, trial and nursing staff worldwide who contributed to the outstanding progress made in the treatment of ALL, and to patients and caregivers who are central to this effort.
REFERENCES
- Gokbuget N, Boissel N, Chiaretti S, et al. Management of ALL in Adults: 2023 ELN Recommendations from a European Expert Panel. Blood. 2024;10.1182/blood.2023023568
- Rousselot P, Coude MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774-82. 10.1182/blood-2016-02-700153
- Short NJ, Kantarjian H, Jabbour E. SOHO State of the Art Updates & Next Questions: Intensive and Non-Intensive Approaches for Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2022;22(2):61-66. 10.1016/j.clml.2021.08.003
- Lennmyr E, Karlsson K, Ahlberg L, et al. Survival in adult acute lymphoblastic leukaemia (ALL): A report from the Swedish ALL Registry. Eur J Haematol. 2019;103(2):88-98. 10.1111/ejh.13247
- Chalandon Y, Rousselot P, Chevret S, et al. Nilotinib with or without cytarabine for Philadelphia positive acute lymphoblastic leukemia. Blood. 2024;10.1182/blood.2023023502
- Kopmar NE, Cassaday RD. How I prevent and treat central nervous system disease in adults with acute lymphoblastic leukemia. Blood. 2023;141(12):1379-1388. 10.1182/blood.2022017035
- Elias Jabbour HMK, Ibrahim Aldoss, Pau Montesinos, Jessica Taft Leonard, David Gomez-Almaguer, Maria R. Baer, Carlo Gambacorti-Passerini, James McCloskey, Yosuke Minami, Cristina Papayannidis, Vanderson Geraldo Rocha, Philippe Rousselot, Pankit Vachhani, Eunice S. Wang, Bingxia Wang, Meliessa Hennessy, Alexander Vorog, Niti Patel, and Josep-Maria Ribera. First report of PhALLCON: A phase 3 study comparing ponatinib (pon) vs imatinib (im) in newly diagnosed patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL). Journal of Clinical Oncology. 2023;41(36)(suppl.398868). https://doi.org/10.1200/JCO.2023.41.36_suppl.398868
- Silva WF, Silverio A, Duarte BKL, et al. Philadelphia-positive B-lymphoblastic leukemia in a middle-income country - A real-world multicenter cohort. Leuk Res. 2021;110:106666. 10.1016/j.leukres.2021.106666
- Short NJ, Kantarjian H, Jabbour E, Ravandi F. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. 2017;30(3):193-200. 10.1016/j.beha.2017.05.001
- Nakasone H, Kako S, Mori T, et al. Stopping tyrosine kinase inhibitors started after allogeneic HCT in patients with Philadelphia chromosome-positive leukemia. Bone Marrow Transplant. 2021;56(6):1402-1412. 10.1038/s41409-020-01206-5
- Saini N, Marin D, Ledesma C, et al. Impact of TKIs post-allogeneic hematopoietic cell transplantation in Philadelphia chromosome-positive ALL. Blood. 8 2020;136(15):1786-1789. 10.1182/blood.2019004685
- Warraich Z, Tenneti P, Thai T, et al. Relapse Prevention with Tyrosine Kinase Inhibitors after Allogeneic Transplantation for Philadelphia Chromosome-Positive Acute Lymphoblast Leukemia: A Systematic Review. Biol Blood Marrow Transplant. Mar 2020;26(3):e55-e64. 10.1016/j.bbmt.2019.09.022
- Pfeifer H, Wassmann B, Bethge W, et al. Randomized comparison of prophylactic and minimal residual disease-triggered imatinib after allogeneic stem cell transplantation for BCR-ABL1-positive acute lymphoblastic leukemia. Leukemia. 2013;27(6):1254-62. 10.1038/leu.2012.352
- Samra B, Kantarjian HM, Sasaki K, et al. Discontinuation of Maintenance Tyrosine Kinase Inhibitors in Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia outside of Transplant. Acta Haematol. 2021;144(3):285-292. 10.1159/000510112
- Ribera JM, Garcia-Calduch O, Ribera J, et al. Ponatinib, chemotherapy, and transplant in adults with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood Adv. 2022;6(18):5395-5402. 10.1182/bloodadvances.2022007764
- Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125(24):3711-9. 10.1182/blood-2015-02-627935
- Short NJ, Kantarjian H, Pui CH, Goldstone A, Jabbour E. SOHO State of the Art Update and Next Questions: Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia. Clin Lymphoma Myeloma Leuk. 2018;18(7):439-446. 10.1016/j.clml.2018.05.015
- Pulsipher MA, Han X, Maude SL, et al. Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov. 2022;3(1):66-81. 10.1158/2643-3230.BCD-21-0095
- Short NJ, Jabbour E, Macaron W, et al. Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol. 2023;98(8):1196-1203. 10.1002/ajh.26949
- Hohtari H, Pallisgaard N, Kankainen M, et al. Copy number alterations define outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2022;107(8):1971-1976. 10.3324/haematol.2021.280578
- Pfeifer H, Raum K, Markovic S, et al. Genomic CDKN2A/2B deletions in adult Ph(+) ALL are adverse despite allogeneic stem cell transplantation. Blood. 2018;131(13):1464-1475. 10.1182/blood-2017-07-796862
- Foa R, Bassan R, Vitale A, et al. Dasatinib-Blinatumomab for Ph-Positive Acute Lymphoblastic Leukemia in Adults. N Engl J Med. 2020;383(17):1613-1623. 10.1056/NEJMoa2016272
- Foa R, Bassan R, Elia L, et al. Long-Term Results of the Dasatinib-Blinatumomab Protocol for Adult Philadelphia-Positive ALL. J Clin Oncol. 2024;42(8):881-885. 10.1200/JCO.23.01075
- Short N, Jabour E, Jain N, Huang X, Macaron W, et al. A chemotherapy-free combination of ponatinib and blinatumomab for patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Hemasphere. 2023;7(Suppl):e4968152. doi: 10.1097/01.HS9.0000967384.49681.52
- Litzow MR, Sun Z, Paietta E, Mattison RJ, Lazarus HM, et al. Consolidation Therapy with Blinatumomab Improves Overall Survival in Newly Diagnosed Adult Patients with B-Lineage Acute Lymphoblastic Leukemia in Measurable Residual Disease Negative Remission: Results from the ECOG-ACRIN E1910 Randomized Phase III National Cooperative Clinical Trials Network Trial. Blood. 2022;140(Supplement 2):LBA-1. doi: https://doi.org/10.1182/blood-2022-171751
- Wieduwilt M, Yin J, Kour O, Teske R, Stock W, et al. Chemotherapy-free treatment with inotuzumab ozogamicin and blinatumomab for older adults with newly diagnosed, Ph-negative, CD22-positive B-cell acute lymphoblastic leukemia: ALLIANCE A041703. Hemasphere. 2023;7(Suppl):e08838b7. doi: 10.1097/01.HS9.0000967380.08838.b7
- Gardner RA, Finney O, Annesley C, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129(25):3322-3331. 10.1182/blood-2017-02-769208
- Hay KA, Gauthier J, Hirayama AV, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019;133(15):1652-1663. 10.1182/blood-2018-11-883710
- Park JH, Riviere I, Gonen M, et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449-459. 10.1056/NEJMoa1709919
- Pasquini MC, Hu ZH, Curran K, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4(21):5414-5424. 10.1182/bloodadvances.2020003092
- Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491-502. 10.1016/S0140-6736(21)01222-8
- Shah BD, Cassaday RD, Park JH, et al. Impact of prior therapies and subsequent transplantation on outcomes in adult patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with brexucabtagene autoleucel in ZUMA-3. J Immunother Cancer. 2023;11(8)10.1136/jitc-2023-007118
- Bader P, Rossig C, Hutter M, et al. CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv. 2023;7(11):2436-2448. 10.1182/bloodadvances.2022008981
- Myers RM, Li Y, Barz Leahy A, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(27):3044-3055. 10.1200/JCO.20.03458
- Benjamin R, Graham C, Yallop D, et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: results of two phase 1 studies. Lancet. 2020;396(10266):1885-1894. 10.1016/S0140-6736(20)32334-5
- Roddie C, Dias J, O'Reilly MA, et al. Durable Responses and Low Toxicity After Fast Off-Rate CD19 Chimeric Antigen Receptor-T Therapy in Adults With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol. 2021;39(30):3352-3363. 10.1200/JCO.21.00917
- Spiegel JY, Patel S, Muffly L, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med. 2021;27(8):1419-1431. 10.1038/s41591-021-01436-0
Table of Contents
©2024 the author(s). Published with license by Medicom Medical Publishers.
This an Open Access article distributed under the terms of the Creative Commons attribution-non Commercial license (http://creativecommons.org/licenses/by-nc/4.0/), which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Posted on
Previous Article
« Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)?
Next Article
Definitions of acute myeloid leukaemia and their clinical significance according to the WHO 2022 and ICC classification »
Related Articles
April 30, 2024
Acute myeloid leukaemia: First debate on leukaemia biology
© 2024 Medicom Medical Publishers. All rights reserved.
Terms and Conditions
| Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com
Table of Contents
« Drug-based maintenance strategies post-allogeneic stem cell transplantation – are we there yet (and will we be)? Next Article
Definitions of acute myeloid leukaemia and their clinical significance according to the WHO 2022 and ICC classification »
Related Articles
April 30, 2024
Acute myeloid leukaemia: First debate on leukaemia biology
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

