Treatment of hand osteoarthritis is complicated as common analgesics and non-steroidal anti-inflammatory drugs (NSAIDs) used for symptomatic relief are often poorly tolerated or contraindicated, especially in elderly patients. At the same time, no effective and proven disease-modifying therapy is available. Dr Claudia Kedor (Charité – Universitätsmedizin Berlin, Germany) presented the results of the randomised, double-blind, placebo controlled, multicentre, investigator-initiated, phase 3 OA-TREAT trial, assessing the efficacy and safety of hydroxychloroquine in patients with inflammatory and erosive hand osteoarthritis.
Patients with inflammatory and erosive hand osteoarthritis were randomised to hydroxychloroquine 200-400 mg per day (n=75) or matching placebo for 52 weeks (n=78). Both groups received standard therapy (stable NSAIDs). The primary endpoint was AUStralian CANadian Osteoarthritis Hand Index (AUSCAN) for pain and hand disability at week 52. The secondary endpoint was radiographic progression from baseline to week 52. Mean age of the patients was 52.4 years in the hydroxychloroquine group and 50.2 years in the placebo group. The percentage of female patients was 90.7% and 76.9%, respectively, and disease duration was 9.5 and 10.8 years, respectively. Baseline pain (AUSCAN) was 31.1 and 30.7, respectively, while baseline function (AUSCAN) was 58.5 and 57.8, respectively.
The results showed that only morning stiffness was significantly reduced in those patients receiving hydroxychloroquine (P=0.001), while changes in radiographic scores did not differ significantly (P>0.05) between both treatment groups (see Table). Regarding safety, 7 serious adverse events were reported in the hydroxychloroquine group versus 15 in the placebo group. No new safety issues were detected. The genesis of stiffness in hand OA could certainly differ from RA and these findings on improvement in stiffness may warrant further investigation.
Table: ANCOVA-adjusted mean values and 95% CI for primary and secondary outcomes at week 52 [1]
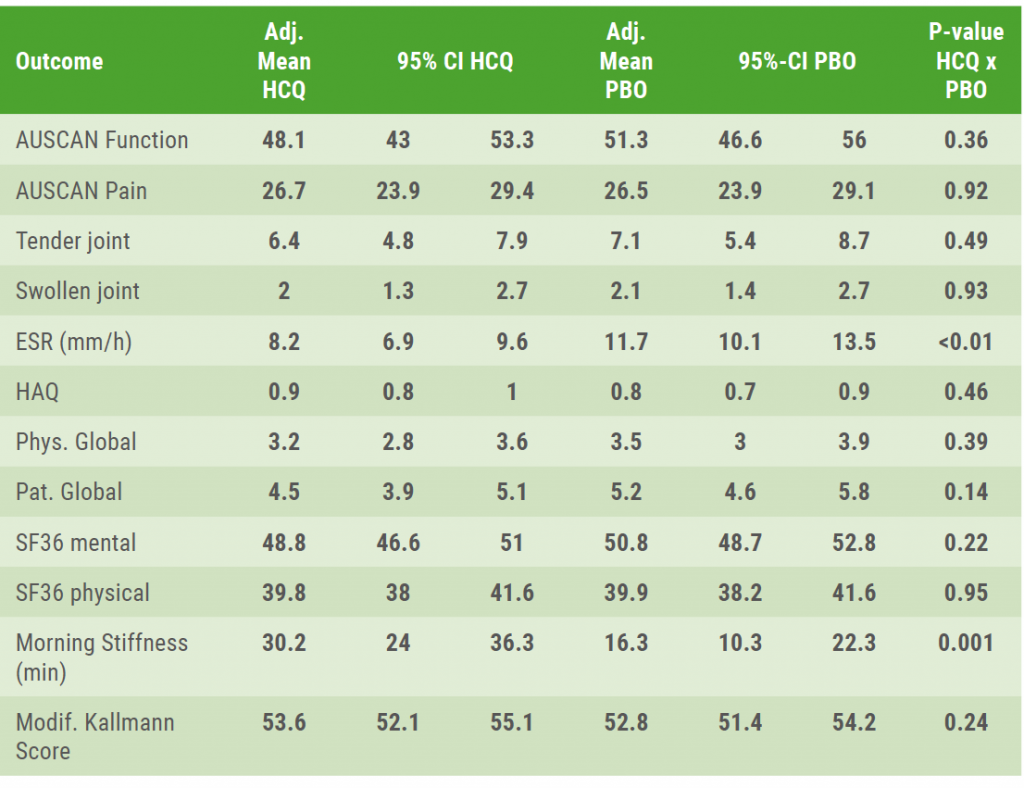 The associated baseline value or, if available, a mean value from baseline and screening was included in the ANCOVA model as a covariate.
The associated baseline value or, if available, a mean value from baseline and screening was included in the ANCOVA model as a covariate.Posted on
Previous Article
« Positive effect denosumab on fall risk Next Article
Higher mortality risk with tramadol versus NSAIDs for osteoarthritis patients »
« Positive effect denosumab on fall risk Next Article
Higher mortality risk with tramadol versus NSAIDs for osteoarthritis patients »
Table of Contents: EULAR 2020
Featured articles
COVID-19 and inflammatory rheumatic disease: some key issues
Secukinumab monotherapy as efficient as adalimumab
AxSpA real-life remission rates higher on biologics
Olokizumab significantly improves RA features and patient-reported outcomes
Rheumatoid Arthritis
New nanoparticle promising future agent in RA
Olokizumab significantly improves RA features and patient-reported outcomes
Low DAS at 4 months predicts sustained DMARD-free remission
Ankylosing Spondylitis
Reduced maintenance dose of certolizumab pegol can be used in axSpA
Worse response axSpA patients to second TNFi versus first TNFi
AxSpA real-life remission rates higher on biologics
Certolizumab pegol reduces acute anterior uveitis in axial spondyloarthritis
TNF-α inhibitors improve bone mineral density in AS patients
Psoriatic Arthritis
Ixekizumab shows sustained improvements in pain and fatigue at 3 years
Adalimumab added to methotrexate yields better results in PsA than methotrexate escalatio
Upadacitinib provides fast onset of improvement in psoriatic arthritis
Secukinumab monotherapy as efficient as adalimumab
Osteoporosis and Osteoarthritis
Higher mortality risk with tramadol versus NSAIDs for osteoarthritis patients
Hydroxychloroquine not effective in patients with hand osteoarthritis
Positive effect denosumab on fall risk
Systemic Sclerosis and Systemic Lupus Erythematosus
Anifrolumab achieves rapid and durable BICLA-response
Subclinical myocardial involvement progresses in SSc patients
Composite endpoint CRESS for primary Sjögren’s syndrome
COVID-19
COVID-19 and inflammatory rheumatic disease: some key issues
Related Articles
December 1, 2022
High retention rates after switching between infliximab biosimilars
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

