The interim analysis of preliminary data from the CONNECT-IBD study showed that safety of CT-P13 is consistent with the known safety profile of infliximab; no new safety information was identified that warrants a change in the benefit-risk profile of CT-P13 [1]. CONNECT-IBD is an ongoing, non-interventional, observational cohort study evaluating CT-P13 in the context of usual care with the infliximab reference product in the treatment of Crohn’s disease (CD) and ulcerative colitis (UC) patients in a real-world setting.
Patients were recruited at 150 academic and community sites in 13 European countries. Adult CD or UC patients prescribed with CT-P13 or EU-sourced infliximab reference product at the investigator’s discretion were eligible. This descriptive analysis included 1,957 patients (CT-P13, n=1,825; Switched, n=132). Of these patients, 1,264 had CD and 692 had UC. In total, 626 treatment-emergent adverse events (AEs) were reported in 438 (22.4%) patients, equally distributed in the CT-P13 (22.2%) and Switched (25.0%) groups. Incidences of treatment-emergent AEs, serious treatment-emergent AEs (12.1% vs 12.1%) and treatment-emergent AEs leading to discontinuation of study drug (8.1% vs 6.8%) were balanced between CT-P13 and Switched groups, respectively. The higher percentage of Switched (2.3%) vs CT-P13 (0.9%) patients withdrawing due to AEs was likely driven by the smaller number of patients in the Switched group. Most patients reported treatment-emergent AEs of mild-to-moderate intensity (overall: mild, 7.3%; moderate, 9.2%; severe, 5.8%).
Preliminary data from the SPOSIB SB2 study on the use of SB2 showed that efficacy and safety seem to be similar overall to those reported for infliximab originator and infliximab biosimilar CT-P13 [2]. SPOSIB SB2 is a multicentre, observational, prospective study of the cohort of the Sicilian Network for Inflammatory Bowel Disease (SN-IBD). All consecutive CD and UC patients starting SB2 from March 2018 to September 2019 are eligible. The primary endpoint is safety. Presented during the congress were the results from the first 6 months of the study, which included 77 patients (CD 50.6%, UC 49.4%). Most patients (n=46; 59.7%) were anti-TNFs naïve, while 66 patients (85.7%) were not previously exposed to infliximab, 8 patients (10.4%) switched from infliximab originator to SB2, and 3 (3.9%) switched from infliximab biosimilar CT-P13 to SB2. The mean follow-up was 2.2 ± 1.7 months. Serious AEs occurred in 7 patients (9.1%); 6 of these led to withdrawal of the drug. In detail, 3 infusion reactions were reported, 3 arthritic flares/arthralgias, and 1 case of flu-like syndrome.
The efficacy of SB2 was evaluated in 35 patients who completed at least 8 weeks of follow-up. Seventeen patients (48.6%) had steroid-free remission after 8 weeks, 8 (22.8%) achieved a partial response, while 10 had no response (28.6%). Among the 25 patients with steroid-free remission or response at week 8, the efficacy rates were 96.6%, 89.1%, and 72.8% after 12, 16, and 20 weeks of therapy, respectively.
1. Bokemeyer B, et al. ECCO 2019, P590.
2. Macaluso FS, et al. ECCO 2019, P737.
Posted on
Previous Article
« p19-Directed IL-23 antibody mirikizumab Next Article
Immune cells and microbes: a happy marriage? »
« p19-Directed IL-23 antibody mirikizumab Next Article
Immune cells and microbes: a happy marriage? »
Table of Contents: ECCO 2019
Featured articles
Interview with Prof. Janneke van der Woude
New Compounds: Study Results
Short-term and Long-term Treatment Results
The right drug for the right patient
Vedolizumab superior to adalimumab in ulcerative colitis
Complementary and Alternative Medicine
Crohn’s disease exclusion diet + partial enteral nutrition in paediatric Crohn’s disease
Microbial composition and psychological wellbeing
Remission
Early remission of Crohn’s disease prevents progression
Proactive adalimumab trough measurements
Observational Studies
IBD risk of treatment with IL-17 antagonists
Basic and Preclinical Research
Immune cells and microbes: a happy marriage?
Genetics
Related Articles
May 9, 2019
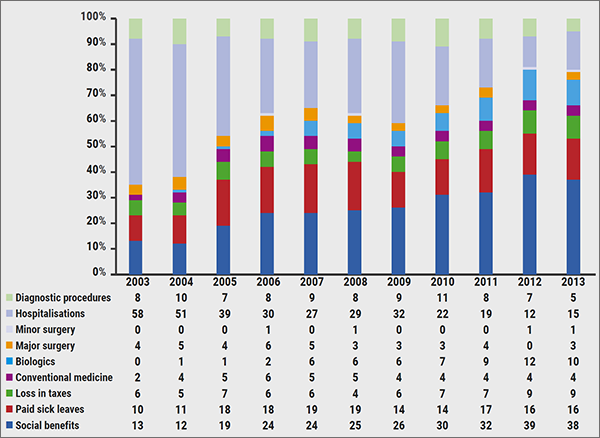
The costs and benefits of biologicals
May 9, 2019
HDAC6 inhibition by CKD-506
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

