A total of 894 patients with UC and 1,349 with CD enrolled in GEMINI long-term safety for a planned treatment duration of 9 years. All patients had received ≥1 prior conventional therapy. Adverse events (AEs) occurred in 93% of UC patients and 96% of CD patients; most frequent were UC (36%) and CD (35%) exacerbations and nasopharyngitis (UC, 28%; CD, 25%). No new trends were observed for infections, malignancies, infusion-related reactions, or hepatic events. Serious AEs were reported in 31% of UC patients and 41% of CD patients; disease exacerbation being the most frequent serious AEs in both cohorts (UC, 13%; CD, 17%). Vedolizumab was discontinued due to AEs in 15% of UC patients and 17% of CD patients; the most frequent reason being UC or CD exacerbation (9% and 8%, respectively). No cases of progressive multi-focal leukoencephalopathy were seen. There were 10 deaths (UC, 4; CD, 6) during the study.
Other results from the GEMINI programme that were presented at ECCO 2019 include the following:
- Long-term treatment with vedolizumab was associated with low immunogenicity rates (consistent with results from GEMINI 1 and 2), even in patients initially treated with vedolizumab induction followed by placebo maintenance in GEMINI 1 and 2 who were subsequently re-treated with vedolizumab in GEMINI long-term safety. No relationship was observed between immunogenicity and safety [2].
- For induction therapy in patients with moderate-to-severe CD, combined therapy with vedolizumab and ongoing corticosteroid may be a synergistic approach, with a similar safety profile, compared with continued corticosteroid use alone or vedolizumab without ongoing corticosteroid use [3].
- Can vedolizumab reduce surgical rates in patients with UC and CD? In an analysis of 834 patients from 3 trials (GEMINI I, III, and long-term safety), surgery rates were lower in the vedolizumab than in the placebo group in the first year of observation. In UC, this difference reached statistical significance. For patients who continued treatment for up to 5 years, vedolizumab provided long-term benefit in both diseases with low rates of surgical intervention [4].
1. Vermeire S, et al. ECCO 2019, OP26.
2. Wyant T, et al. ECCO 2019, P441.
3. Sands E, et al. ECCO 2019, DOP054.
4. Feagan BG, et al. ECCO 2019, DOP79.
Posted on
Previous Article
« Crohn’s disease exclusion diet + partial enteral nutrition in paediatric Crohn’s disease Next Article
Selective oral sphingosine 1-phosphate receptor modulator amiselimod »
« Crohn’s disease exclusion diet + partial enteral nutrition in paediatric Crohn’s disease Next Article
Selective oral sphingosine 1-phosphate receptor modulator amiselimod »
Table of Contents: ECCO 2019
Featured articles
Interview with Prof. Janneke van der Woude
New Compounds: Study Results
Short-term and Long-term Treatment Results
The right drug for the right patient
Vedolizumab superior to adalimumab in ulcerative colitis
Complementary and Alternative Medicine
Crohn’s disease exclusion diet + partial enteral nutrition in paediatric Crohn’s disease
Microbial composition and psychological wellbeing
Remission
Early remission of Crohn’s disease prevents progression
Proactive adalimumab trough measurements
Observational Studies
IBD risk of treatment with IL-17 antagonists
Basic and Preclinical Research
Immune cells and microbes: a happy marriage?
Genetics
Related Articles
May 9, 2019
Immune cells and microbes: a happy marriage?
May 9, 2019
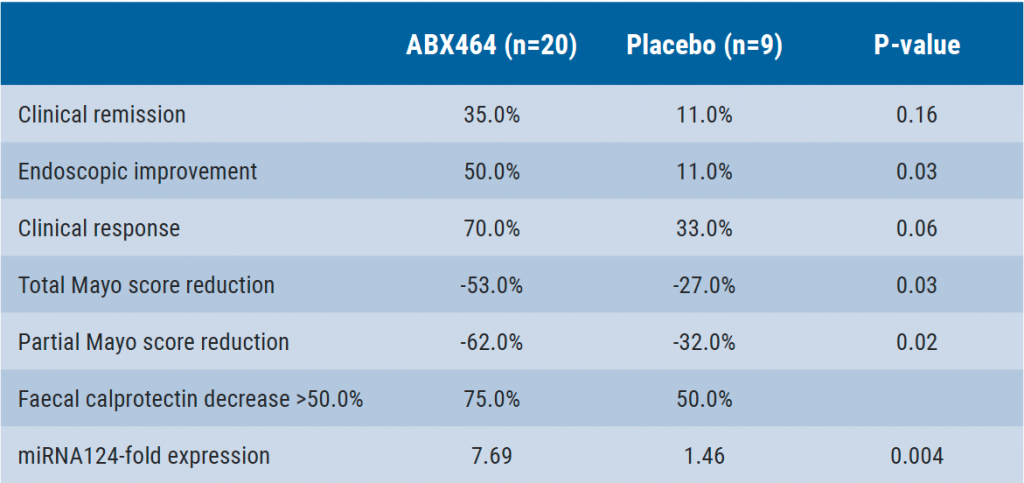
ABX464: HIV drug tested in ulcerative colitis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

