The study was performed in 32 patients from 15 European centres. A total of 29 (90.6%) patients (20 randomised to ABX464 and 9 to placebo) completed the induction study. The overall safety of ABX464 was very good, with no serious adverse events. The safety profile was similar to that seen in the clinical development in the HIV indication. The main efficacy results are presented in the Table.
Table: ABX464-101 study endpoints results after 56 days [1]
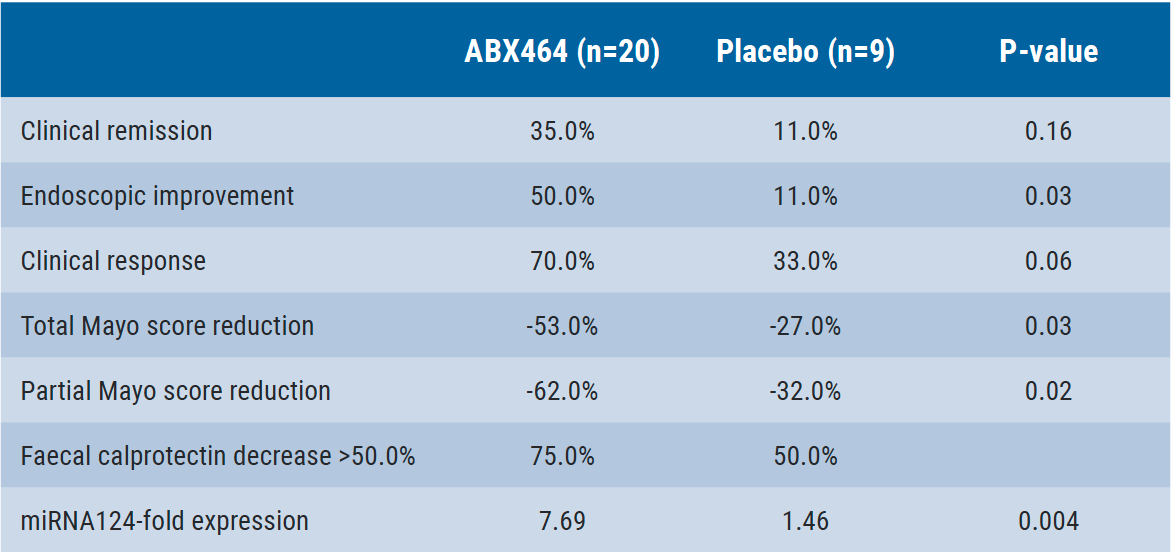
After the blinded induction phase, patients had the option to enrol in a 52-week, open-label, 50 mg once daily ABX464 study. The interim data from this maintenance study (n=22) shows further improvement in partial Mayo score and reduction in faecal calprotectin. This data supports a phase 2b multicentre, placebo-controlled, dose-ranging study in ulcerative colitis and a phase 2a study in Crohn’s disease.
- Vermeire S, et al. ECCO 2019, OP21.
Posted on
Previous Article
« Cyclosporine: novel low-dose, controlled-release formulation Next Article
Limited long-term effectiveness and safety of tacrolimus in ulcerative colitis »
« Cyclosporine: novel low-dose, controlled-release formulation Next Article
Limited long-term effectiveness and safety of tacrolimus in ulcerative colitis »
Table of Contents: ECCO 2019
Featured articles
Interview with Prof. Janneke van der Woude
New Compounds: Study Results
Short-term and Long-term Treatment Results
The right drug for the right patient
Vedolizumab superior to adalimumab in ulcerative colitis
Complementary and Alternative Medicine
Crohn’s disease exclusion diet + partial enteral nutrition in paediatric Crohn’s disease
Microbial composition and psychological wellbeing
Remission
Early remission of Crohn’s disease prevents progression
Proactive adalimumab trough measurements
Observational Studies
IBD risk of treatment with IL-17 antagonists
Basic and Preclinical Research
Immune cells and microbes: a happy marriage?
Genetics
Related Articles
May 9, 2019
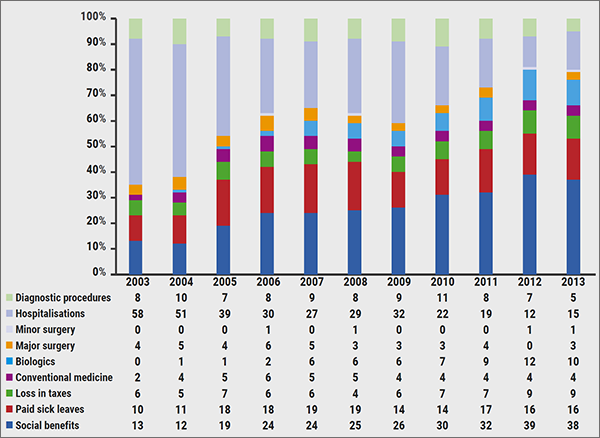
The costs and benefits of biologicals
May 9, 2019
Pathogenesis
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy

