https://doi.org/10.55788/ce33490e
The sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin was assessed in the EMPA-KIDNEY trial (NCT03594110) to further investigate the effects of empagliflozin in patients with CKD at risk for disease progression. Dr William Herrington (University of Oxford, UK) presented the results of this randomised, parallel-group, double-blind, placebo-controlled study. Eligible patients had an estimated glomerular filtration rate (eGFR) of 20–44 mL/min/1.73m2 with no minimum level of albuminuria or an eGFR of 45–89 mL/min/1.73m2 with a urine albumin-to-creatinine ratio ≥200 mg/g. In total, 6,009 patients were randomised 1:1 to either empagliflozin 10 mg daily (n=3,304) or matching placebo (n=3,305). The primary outcome of the study was a composite of progression of kidney disease (defined as end-stage renal disease, a sustained decrease in eGFR to <10 mL/min/1.73m2, a sustained decrease in eGFR ≥40% from baseline, or death from renal causes) or death from CV causes. Median follow-up was 2 years, mean age of patients was 64 years, and 33% were female. Progression of kidney disease or death from CV causes occurred in 13.1% of patients in the empagliflozin group versus 16.9% of patients in the placebo group (HR 0.72; 95% CI 0.64–0.82; P<0.001) (see Figure). This outcome means a significant 28% RR reduction in the primary combined endpoint compared with placebo. The efficacy of empagliflozin was similar irrespective of whether patients had type 2 diabetes at the time of enrolment and of their eGFR at entry. Hospitalisation for any cause was lower with empagliflozin than with placebo (24.8 events/100 patient-years vs 29.2 events/100 person-years, respectively; P=0.003), whereas the proportion of patients who experienced hospitalisation for heart failure or death from CV causes was 4.0% versus 4.6% respectively (P=0.15), death from any cause was 4.5% versus 5.1% of patients, respectively (P=0.21), and progression of kidney disease was 11.6% versus 15.2%, respectively (HR 0.71; 95% CI 0.62–0.81) [1, 2].
Figure: Primary composite outcome: Progression of kidney disease or death from CV causes.
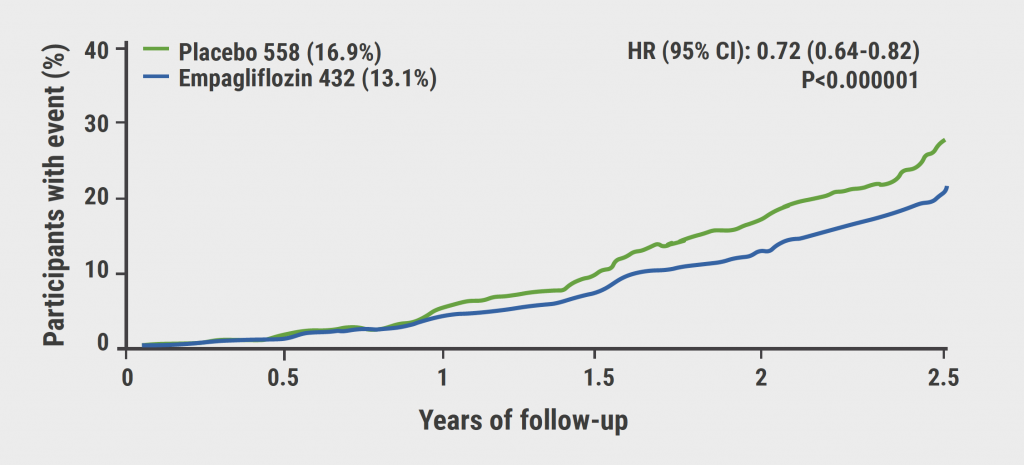
Reprinted from The EMPA-KIDNEY Collaborative Group. New Engl J Med. 2022 Nov 4. DOI: 10.1056/NEJMoa2204233. Copyright 2022, with permission from Massachusetts Medical Society.
In addition, a summary data meta-analysis of the effects of SGLT2 inhibitors on a common composite of kidney disease progression outcome and acute kidney injury events including 90,409 participants worldwide showed that SGLT2 inhibitors reduce the risk of kidney disease progression and AKI safely irrespective of diabetes status. “A primary kidney diagnosis does not appear to modify these relative benefits,” Dr Nathalie Staplin (University of Oxford, UK) said. “The absolute benefits clearly exceed the harm on patients with CKD irrespective of diabetes status [3, 4].”
- Herrington WG, et al. Empagliflozin in Patients With CKD. FR-OR68 ASN Kidney Week 2022, 3–6 Nov.
- The EMPA-KIDNEY Collaborative Group. N Engl J Med, Nov 4, 2022.
- Staplin N, et al. Sodium Glucose Cotransporter-2 (SGLT2) Inhibitors Among Patients with and without Diabetes: Collaborative Meta-Analysis of Large Placebo-Controlled Trials. FR-069, ASN Kidney Week 2022, 3–6 Nov.
- The Nuffield Department of Population Health Renal Studies Group. Lancet 2022;400:1788–1801.
Copyright ©2022 Medicom Medical Publishers
Posted on
Previous Article
« Combining UACR and GFR improves prediction of drug effect in CKD phase 2 trials Next Article
VALOR-CKD trial did not show any benefits for veverimer »
« Combining UACR and GFR improves prediction of drug effect in CKD phase 2 trials Next Article
VALOR-CKD trial did not show any benefits for veverimer »
Table of Contents: ASN 2022
Featured articles
Chronic Kidney Disease
VALOR-CKD trial did not show any benefits for veverimer
EMPA-KIDNEY: empagliflozin slashes kidney disease progression or CV death
Combining UACR and GFR improves prediction of drug effect in CKD phase 2 trials
Dapagliflozin reduces number of hospitalisations in patients with CKD
Novel MSC therapy appears safe and effective in preventing decline in eGFR
Dapagliflozin improves anaemia in patients with CKD with or without T2D
Kidney Transplantation and Dialysis
Balanced crystalloid solution better for deceased donor kidney transplantations
Modified donor blood cells seem a promising option in kidney transplant recipients
Cooler dialysate does not offer any clinical benefits
Antiviral effect of MAU868 against BK virus prompts further research
General Nephrology
Medication-targeted alerts for the risk of AKI
Coaching with a DASH diet improves albuminuria
Cemdisiran shows promise in IgA nephropathy
Long-term nephroprotective effects of sparsentan in FSGS
Encaleret normalises mineral homeostasis in patients with ADH1
Adding voclosporin to MMF and steroids results in long-term higher CRR in severe lupus nephritis
Selonsertib poses risk of AKI in patients with DKD
Significantly higher risk of overcorrection in hyponatraemic patients with standard bolus infusion
Lowering blood pressure intervention favourable for CV outcomes
Related Articles
© 2024 Medicom Medical Publishers. All rights reserved. Terms and Conditions | Privacy Policy
HEAD OFFICE
Laarderhoogtweg 25
1101 EB Amsterdam
The Netherlands
T: +31 85 4012 560
E: publishers@medicom-publishers.com

